Genomic Medicine and Policy
(Closed)
Share This Topical Collection
Editors
 Dr. Christine Lu
Dr. Christine Lu
 Dr. Christine Lu
Dr. Christine Lu
E-Mail
Collection Editor
Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA
Interests: clinical implementation of precision medicine; clinical and economic outcomes related to the use of precision medicine, including the impact of value-based contracts; policy, legal, ethical, economic and societal issues of precision medicine in clinical practice, including disparities in access to precision medicine and outcomes, and ethical implications
Special Issues, Collections and Topics in MDPI journals
 Dr. Kurt Christensen
Dr. Kurt Christensen
 Dr. Kurt Christensen
Dr. Kurt Christensen
E-Mail
Website
Collection Editor
1. Division of Genetics, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
2. Harvard Medical School, Boston, MA, USA
Interests: medical, behavioral and economic impact of genomic tests, with a focus on asymptomatic populations and methods of implementation
 Dr. Nina Sperber
Dr. Nina Sperber
 Dr. Nina Sperber
Dr. Nina Sperber
E-Mail
Website
Collection Editor
1. Durham VA Health Care System, Durham, NC, USA
2. Department of Population Health Sciences, Duke University School of Medicine, Durham, NC, USA
Interests: genomics; clinical decision support; clinical informatics; implementation science
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The rapid development of genomic technologies has generated important questions for policy makers, health system leaders, laboratories, clinicians, patients, and the public. The Journal of Personalized Medicine aims to publish a collection of articles that explore the legal, ethical and economic challenges and opportunities of the field and provide fresh insights to ongoing discussions and debate in genomic medicine. We will consider original research, systematic reviews, and well-designed case studies and analyses that report empirical work that presents experiences and perspectives from the US and abroad.
The included topics in the issue are:
- Policy analyses of genomic medicine, including data privacy and sharing of results
- Ethical frameworks for genomic information disclosure, such as the disclosure of secondary findings in pediatric populations
- Comparative effectiveness of genomic medicine
- Economic evaluations of genomic medicine, including methods and challenges
- Implementation of genomic medicine in diverse health care settings, including methods and challenges
- Pricing, coverage and reimbursement of genomic medicine
Dr. Christine Lu
Dr. Kurt Christensen
Dr. Nina Sperber
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Personalized Medicine is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Policy
- Legal
- Ethical
- Economic
- Precision Medicine
- Reimbursement
- Genomic testing
Published Papers (12 papers)
Open AccessArticle
Exploring Implementation of Personal Breast Cancer Risk Assessments
by
Maria A. Sierra, Jack C. W. Wheeler, Lisa Devereux, Alison H. Trainer and Louise Keogh
Cited by 9 | Viewed by 2380
Abstract
Personal Breast Cancer (BC) Risk Assessments (PBCRA) have potential to stratify women into clinically-actionable BC risk categories. As this could involve population-wide genomic testing, women’s attitudes to PBCRA and views on acceptable implementation platforms must be considered to ensure optimal population participation. We
[...] Read more.
Personal Breast Cancer (BC) Risk Assessments (PBCRA) have potential to stratify women into clinically-actionable BC risk categories. As this could involve population-wide genomic testing, women’s attitudes to PBCRA and views on acceptable implementation platforms must be considered to ensure optimal population participation. We explored these issues with 31 women with different BC risk profiles through semi-structured focus group discussions or interviews. Inductive thematic coding of transcripts was performed. Subsequently, women listed factors that would impact on their decision to participate. Participants’ attitudes to PBCRA were positive. Identified themes included that PBCRA acceptance hinges on result actionability. Women value the ability to inform decision-making. Participants reported anxiety, stress, and genetic discrimination as potential barriers. The age at which PBCRA was offered, ease of access, and how results are returned held importance. Most women value the opportunity for PBCRA to inform increased surveillance, while highlighting hesitance to accept reduced surveillance as they find reassurance in regular screening. Women with
BRCA pathogenic variants value the potential for PBCRA to identify a lower cancer risk and potentially inform delayed prophylactic surgery. This study highlights complexities in adopting advances in BC early detection, especially for current users who value existing processes as a social good.
Full article
Open AccessArticle
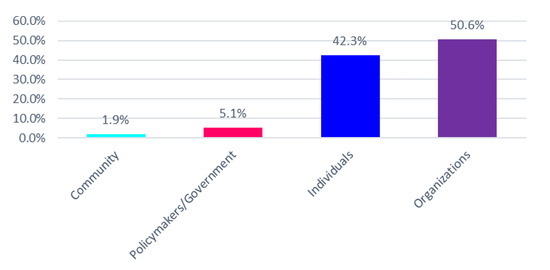
A Qualitative Study to Develop a Privacy and Nondiscrimination Best Practice Framework for Personalized Wellness Programs
by
Rachele M. Hendricks-Sturrup, Kathy L. Cerminara and Christine Y. Lu
Cited by 6 | Viewed by 4886
Abstract
Employers in the United States (US) increasingly offer personalized wellness products as a workplace benefit. In doing so, those employers must be cognizant of not only US law but also European Union (EU) law to the extent that the EU law applies to
[...] Read more.
Employers in the United States (US) increasingly offer personalized wellness products as a workplace benefit. In doing so, those employers must be cognizant of not only US law but also European Union (EU) law to the extent that the EU law applies to European immigrants or guest workers in the US. To the extent that wellness programs are implemented in either public health or employment contexts within the US and/or EU, sponsors of these programs can partner with direct-to-consumer (DTC) genetic testing companies and other digital health companies to generate, collect, and process sensitive health information that are loosely or partially regulated from a privacy and nondiscrimination standpoint. Balancing claims about the benefits of wellness programs are concerns about employee health privacy and discrimination and the current unregulated nature of consumer health data. We qualitatively explored the concerns and opinions of public and legislative stakeholders in the US to determine key themes and develop privacy and nondiscrimination best practices. Key themes emerged as promoting a culture of trust and wellness. Best practices within these themes were: (1) have transparent and prominent data standards and practices, (2) uphold employee privacy and nondiscrimination standards, (3) remove penalties associated with biometric outcomes and nondisclosure of sensitive health information, (4) reward healthy behavior regardless of biometric outcomes, and (5) make program benefits accessible regardless of personal status. Employers, DTC genetic testing companies, policymakers, and stakeholders broadly should consider these themes and best practices in the current absence of broad regulations on nondiscriminatory workplace wellness programs.
Full article
►▼
Show Figures
Open AccessArticle
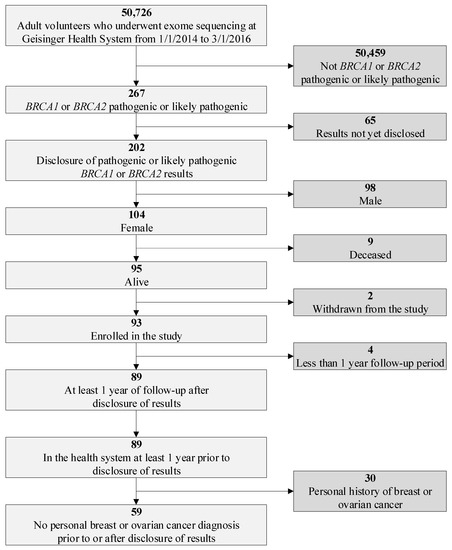
Healthcare Utilization and Costs after Receiving a Positive BRCA1/2 Result from a Genomic Screening Program
by
Jing Hao, Dina Hassen, Kandamurugu Manickam, Michael F. Murray, Dustin N. Hartzel, Yirui Hu, Kunpeng Liu, Alanna Kulchak Rahm, Marc S. Williams, Amanda Lazzeri, Adam Buchanan, Amy Sturm and Susan R. Snyder
Cited by 15 | Viewed by 5626
Abstract
Population genomic screening has been demonstrated to detect at-risk individuals who would not be clinically identified otherwise. However, there are concerns about the increased utilization of unnecessary services and the associated increase in costs. The objectives of this study are twofold: (1) determine
[...] Read more.
Population genomic screening has been demonstrated to detect at-risk individuals who would not be clinically identified otherwise. However, there are concerns about the increased utilization of unnecessary services and the associated increase in costs. The objectives of this study are twofold: (1) determine whether there is a difference in healthcare utilization and costs following disclosure of a pathogenic/likely pathogenic (P/LP)
BRCA1/2 variant via a genomic screening program, and (2) measure the post-disclosure uptake of National Comprehensive Cancer Network (NCCN) guideline-recommended risk management. We retrospectively reviewed electronic health record (EHR) and billing data from a female population of
BRCA1/2 P/LP variant carriers without a personal history of breast or ovarian cancer enrolled in Geisinger’s MyCode genomic screening program with at least a one-year post-disclosure observation period. We identified 59 women for the study cohort out of 50,726 MyCode participants. We found no statistically significant differences in inpatient and outpatient utilization and average total costs between one-year pre- and one-year post-disclosure periods ($18,821 vs. $19,359,
p = 0.76). During the first year post-disclosure, 49.2% of women had a genetic counseling visit, 45.8% had a mammography and 32.2% had an MRI. The uptake of mastectomy and oophorectomy was 3.5% and 11.8%, respectively, and 5% of patients received chemoprevention.
Full article
►▼
Show Figures
Open AccessArticle
Physician Experience with Direct-To-Consumer Genetic Testing in Kaiser Permanente
by
M. Cabell Jonas, Pim Suwannarat, Andrea Burnett-Hartman, Nikki Carroll, Michelle Turner, Kristen Janes, Christine Truong, Erica Blum-Barnett, Nazneen Aziz and Elizabeth A. McGlynn
Cited by 16 | Viewed by 6175
Abstract
Health systems and physicians nationwide aspire to consistently and reliably apply genetic and genomic information to guide disease prevention, management, and treatment. However, clinical information, including genetics/genomics data from within and outside of the care delivery system, is expanding rapidly. Between November 2017
[...] Read more.
Health systems and physicians nationwide aspire to consistently and reliably apply genetic and genomic information to guide disease prevention, management, and treatment. However, clinical information, including genetics/genomics data from within and outside of the care delivery system, is expanding rapidly. Between November 2017 and April 2018, we surveyed 1502 Permanente Medical Group primary care and specialist physicians to assess the degree to which direct-to-consumer genetic test results were being presented to physicians and identify genetics educational needs among physicians (response rate 15%). Adjusted logistic regression (according to respondent characteristics) was used to calculate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) comparing responses within groups. Results showed 35% and 12% of respondents reported receiving at least one direct-to-consumer health risk genetic result (DTC-health risk) or direct-to-consumer pharmacogenomic test result (DTC-PGx), respectively, from a patient in the past year. Of those receiving at least one test result, 40% (DTC-health risk) and 39% (DTC-PGx) of physicians reported 1+ referral(s); 78% (DTC-health risk) and 42% (DTC-PGx) of referrals were to clinical genetics. In total, 85% of physicians would spend ≥2 h/year on genetics/genomics education.
Full article
Open AccessArticle
Access to Genetic Counselors in the Southern United States
by
Catalina Villegas and Susanne B. Haga
Cited by 35 | Viewed by 9040
Abstract
The expansion of genetic and genomic testing across medical specialties and the changing workforce demographics of certified genetic counselors (CGCs) have led to concerns of a workforce shortage. We assessed the number of genetic counselors working in the Southern United States—a rural and
[...] Read more.
The expansion of genetic and genomic testing across medical specialties and the changing workforce demographics of certified genetic counselors (CGCs) have led to concerns of a workforce shortage. We assessed the number of genetic counselors working in the Southern United States—a rural and medically underserved region—using various online and professional resources. We identified 683 practicing genetic counselors across the Southern U.S. and 160 specializing in prenatal genetics. CGCs were concentrated in urban areas; counties with a CGC had a significantly higher proportion of minority residents and median household income than counties without a CGC. There is an average of 2.97 prenatal CGCs per 5000 high-risk births in the South. Alternative delivery models are needed to increase access to counseling services in the Southern U.S., particularly for low income households and those of high risk pregnancies. Increased provider education and patient educational materials can help facilitate informed decision-making in prenatal settings as genetic technologies gain a stronger foothold and bring value to medical practice.
Full article
►▼
Show Figures
Open AccessReview
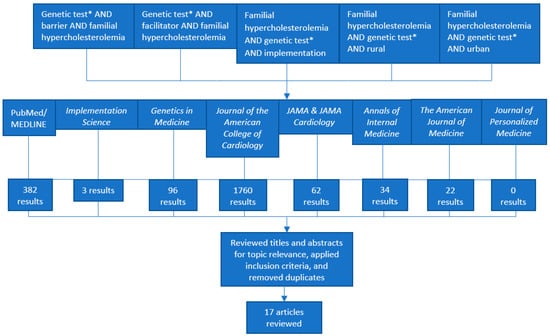
Barriers and Facilitators to Genetic Testing for Familial Hypercholesterolemia in the United States: A Review
by
Rachele M. Hendricks-Sturrup, Kathleen M. Mazor, Amy C. Sturm and Christine Y. Lu
Cited by 23 | Viewed by 8717
Abstract
Familial Hypercholesterolemia (FH) is an underdiagnosed condition in the United States (US) and globally, affecting an estimated 1/250 individuals. It is a genetic risk factor for premature cardiovascular disease and is responsible for an estimated 600,000 to 1.2 million preventable vascular events. Studies
[...] Read more.
Familial Hypercholesterolemia (FH) is an underdiagnosed condition in the United States (US) and globally, affecting an estimated 1/250 individuals. It is a genetic risk factor for premature cardiovascular disease and is responsible for an estimated 600,000 to 1.2 million preventable vascular events. Studies show that FH genetic testing can identify a causal gene variant in 60 to 80% of clinically suspected FH cases. However, FH genetic testing is currently underutilized in clinical settings in the US despite clinical recommendations and evidence supporting its use. Reasons for underutilization are not well understood. We conducted a literature review in the PubMed/MEDLINE database and eight peer-reviewed journals. After filtering for and reviewing 2340 articles against our inclusion criteria, we included nine commentaries or expert opinions and eight empirical studies reported between January 2014 and March 2019 in our review. After applying the Consolidated Framework for Implementation Research (CFIR), we identified a total of 26 potential barriers and 15 potential facilitators (estimated barrier to facilitator ratio of 1.73). We further estimated ratios of potential barriers to facilitators for each CFIR domain (Characteristics of Intervention, Outer Setting, Inner Setting, Characteristics of Individuals, and Process). Findings derived from our systematic approach to the literature and calculations of estimated baseline ratios of barriers and facilitators can guide future research to understand FH genetic testing implementation in diverse clinical settings. Our systematic approach to the CFIR could also be used as a model to understand or compare barriers and facilitators to other evidence-based genetic testing processes in health care settings in the US and abroad.
Full article
►▼
Show Figures
Open AccessArticle
Primary Care Physicians’ Knowledge, Attitudes, and Experience with Personal Genetic Testing
by
Susanne B. Haga, Esther Kim, Rachel A. Myers and Geoffrey S. Ginsburg
Cited by 64 | Viewed by 11928
Abstract
Primary care providers (PCPs) will play an important role in precision medicine. However, their lack of training and knowledge about genetics and genomics may limit their ability to advise patients or interpret or utilize test results. We evaluated PCPs’ awareness of the role
[...] Read more.
Primary care providers (PCPs) will play an important role in precision medicine. However, their lack of training and knowledge about genetics and genomics may limit their ability to advise patients or interpret or utilize test results. We evaluated PCPs’ awareness of the role of genetics/genomics in health, knowledge about key concepts in genomic medicine, perception/attitudes towards direct-to-consumer (DTC) genetic testing, and their level of confidence/comfort in discussing testing with patients prior to and after undergoing DTC testing through the 23andMe Health + Ancestry Service. A total of 130 PCPs completed the study. Sixty-three percent were board-certified in family practice, 32% graduated between 1991 and 2000, and 88% had heard of 23andMe prior to the study. Seventy-two percent decided to participate in the study to gain a better understanding about testing. At baseline, 23% of respondents indicated comfort discussing genetics as a risk factor for common diseases, increasing to 59% after undergoing personal genetic testing (PGT) (
p < 0.01). In summary, we find that undergoing PGT augments physicians’ confidence, comfort, and interest in DTC testing.
Full article
Open AccessCommunication
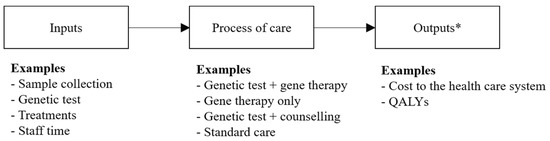
Assessing the Joint Value of Genomic-Based Diagnostic Tests and Gene Therapies
by
Sean P. Gavan, Christine Y. Lu and Katherine Payne
Cited by 7 | Viewed by 7109
Abstract
Gene therapy is an emerging type of treatment that may aim to provide a cure to individuals with a genetic mutation known to be causative of a specific disease. A diagnosis of the causative mutation must precede treatment with a in vivo gene
[...] Read more.
Gene therapy is an emerging type of treatment that may aim to provide a cure to individuals with a genetic mutation known to be causative of a specific disease. A diagnosis of the causative mutation must precede treatment with a in vivo gene therapy. Both achieving a genomic-based diagnosis and treatment with a gene therapy may result in substantial expenditures for health care systems. Uncertainties around the health care costs, risks, and benefits derived from diagnosis and treatment with a subsequent gene therapy suggests a need for developing an evidence base, underpinned by opportunity cost, to inform if, and how, these health technologies should be introduced into health care systems funded by finite budgets. This article discusses why current methods to evaluate health technologies (decision-analytic model-based cost-effectiveness analysis from the perspective of a health care system over a lifetime time horizon) are appropriate to quantify the costs and consequences of using genomic-based diagnostic tests and gene therapies in combination, rather than as separate interventions, within clinical practice. Evaluating the economic impact of test-and-treatment strategies will ensure that the opportunity cost of these health technologies is quantified fully for decision-makers who are responsible for allocating limited resources in health care systems.
Full article
►▼
Show Figures
Open AccessArticle
Exploring Predictors of Genetic Counseling and Testing for Hereditary Breast and Ovarian Cancer: Findings from the 2015 U.S. National Health Interview Survey
by
Caitlin G. Allen, Megan Roberts and Yue Guan
Cited by 20 | Viewed by 6798
Abstract
Despite efforts to increase the availability of clinical genetic testing and counseling for Hereditary Breast and Ovarian (HBOC)-related cancers, these services remain underutilized in clinical settings. There have been few efforts to understand the public’s use of cancer genetic services, particularly for HBOC-related
[...] Read more.
Despite efforts to increase the availability of clinical genetic testing and counseling for Hereditary Breast and Ovarian (HBOC)-related cancers, these services remain underutilized in clinical settings. There have been few efforts to understand the public’s use of cancer genetic services, particularly for HBOC-related cancers. This analysis is based on data from the 2015 National Health Interview Survey (NHIS), a U.S.-based nationwide probability sample, to better understand the public’s use of HBOC-related clinical cancer genetic services. Bivariate analyses were used to compute percentages and examine the associations of familial cancer risk for three genetic services outcomes (ever had genetic counseling for cancer risk, ever discussed genetic testing for cancer risk with a provider, and ever had genetic testing for cancer risk). Multivariable logistic regression models were used to estimate the association of familial cancer risk and other demographic and health variables with genetic services. Most women (87.67%) in this study were at low risk based on self-reported family history of breast and ovarian cancer, 10.65% were at medium risk, and 1.68% were at high risk. Overall, very small numbers of individuals had ever had genetic counseling (2.78%), discussed genetic testing with their physician (4.55%) or had genetic testing (1.64%). Across all genetic services outcomes, individuals who were at higher familial risk were more likely to have had genetic counseling than those at lower risk (high risk: aOR = 5.869, 95% CI = 2.911–11.835; medium risk: aOR = 4.121, 95% CI = 2.934–5.789), discussed genetic testing (high risk: aOR = 5.133, 95% CI = 2.699–9.764; medium risk: aOR = 3.649, 95% CI = 2.696–4.938), and completed genetic testing (high risk: aOR = 8.531, 95% CI = 3.666–19.851; medium risk aOR = 3.057, 95% CI = 1.835–5.094). Those who perceived themselves as being more likely to develop cancer than the average woman were more likely to engage in genetic counseling (aOR = 1.916, 95% CI = 1.334–2.752), discuss genetic testing (aOR = 3.314, 95% CI = 2.463–4.459) or have had genetic testing (aOR = 1.947, 95% CI = 1.13–3.54). Personal cancer history was also a significant predictor of likelihood to have engaged in genetic services. Our findings highlight: (1) potential under-utilization of cancer genetic services among high risk populations in the U.S. and (2) differences in genetic services use based on individual’s characteristics such as self-reported familial risk, personal history, and beliefs about risk of cancer. These results align with other studies which have noted that awareness and use of genetic services are low in the general population and likely not reaching individuals who could benefit most from screening for inherited cancers. Efforts to promote public awareness of familial cancer risk may lead to better uptake of cancer genetic services.
Full article
Open AccessCommentary
Direct-to-Consumer Genetic Testing Data Privacy: Key Concerns and Recommendations Based on Consumer Perspectives
by
Rachele M. Hendricks-Sturrup and Christine Y. Lu
Cited by 23 | Viewed by 11952
Abstract
Direct-to-consumer genetic testing (DTC-GT) companies are engaging health consumers in unprecedented ways and leveraging the genetic information they collect to further engage health companies. This has produced controversy about DTC-GT consumer expectations, standards, and perceptions of privacy. In this commentary, we highlight recent
[...] Read more.
Direct-to-consumer genetic testing (DTC-GT) companies are engaging health consumers in unprecedented ways and leveraging the genetic information they collect to further engage health companies. This has produced controversy about DTC-GT consumer expectations, standards, and perceptions of privacy. In this commentary, we highlight recent events involving DTC-GT companies and controversy about privacy that followed those events and discuss recent studies that have explored DTC-GT consumer concerns about privacy. We discuss DTC-GT company standards of upholding consumer privacy and the general accessibility of DTC-GT company terms of use agreements and privacy policies that are written at reading levels above that of many consumers. We conclude that broader discussions and more research are needed to identify DTC-GT consumer concerns about and expectations of privacy. We anticipate that our recommendations will advance discussions on consumer privacy expectations and protections in an era of increasing engagement in DTC-GT.
Full article
Open AccessCommentary
Understanding Implementation Challenges to Genetic Testing for Familial Hypercholesterolemia in the United States
by
Rachele M. Hendricks-Sturrup and Christine Y. Lu
Cited by 14 | Viewed by 7536
Abstract
Cardiovascular disease (CVD) is the leading cause of death in the United States (US), with familial hypercholesterolemia (FH) being a major inherited and genetic risk factor for premature CVD and atherosclerosis. Genetic testing has helped patients and providers confirm the presence of known
[...] Read more.
Cardiovascular disease (CVD) is the leading cause of death in the United States (US), with familial hypercholesterolemia (FH) being a major inherited and genetic risk factor for premature CVD and atherosclerosis. Genetic testing has helped patients and providers confirm the presence of known pathogenic and likely pathogenic variations in FH-associated genes. Key organizations, such as the Centers for Disease Control and Prevention (CDC), American Heart Association (AHA), FH Foundation, and National Lipid Association (NLA), have recognized the clinical utility of FH genetic testing. However, FH genetic testing is underutilized in clinical practice in the US for reasons that are underexplored through the lens of implementation science. In this commentary, we discuss seven key implementation challenges that must be overcome to strengthen the clinical adoption of FH genetic testing in the US. These implementation challenges center on evidence of cost-effectiveness, navigating patient and provider preferences and concerns, gender and ethnic diversity and representation in genetic testing, and establishing clinical consensus around FH genetic testing based on the latest and most relevant research findings. Overcoming these implementation challenges is imperative to the mission of reducing CVD risk in the US.
Full article
Open AccessArticle
Physician-Reported Benefits and Barriers to Clinical Implementation of Genomic Medicine: A Multi-Site IGNITE-Network Survey
by
Aniwaa Owusu Obeng, Kezhen Fei, Kenneth D. Levy, Amanda R. Elsey, Toni I. Pollin, Andrea H. Ramirez, Kristin W. Weitzel and Carol R. Horowitz
Cited by 100 | Viewed by 10819
Abstract
Genetic medicine is one of the key components of personalized medicine, but adoption in clinical practice is still limited. To understand potential barriers and provider attitudes, we surveyed 285 physicians from five Implementing GeNomics In pracTicE (IGNITE) sites about their perceptions as to
[...] Read more.
Genetic medicine is one of the key components of personalized medicine, but adoption in clinical practice is still limited. To understand potential barriers and provider attitudes, we surveyed 285 physicians from five Implementing GeNomics In pracTicE (IGNITE) sites about their perceptions as to the clinical utility of genetic data as well as their preparedness to integrate it into practice. These responses were also analyzed in comparison to the type of study occurring at the physicians’ institution (pharmacogenetics versus disease genetics). The majority believed that genetic testing is clinically useful; however, only a third believed that they had obtained adequate training to care for genetically “high-risk” patients. Physicians involved in pharmacogenetics initiatives were more favorable towards genetic testing applications; they found it to be clinically useful and felt more prepared and confident in their abilities to adopt it into their practice in comparison to those participating in disease genetics initiatives. These results suggest that investigators should explore which attributes of clinical pharmacogenetics (such as the use of simplified genetics-guided recommendations) can be implemented to improve attitudes and preparedness to implement disease genetics in care. Most physicians felt unprepared to use genetic information in their practice; accordingly, major steps should be taken to develop effective clinical tools and training strategies for physicians.
Full article