Novel Approache of Anticancer Therapy
(Closed)
Share This Topical Collection
Editor
 Dr. Isabelle Mus-Veteau
Dr. Isabelle Mus-Veteau
 Dr. Isabelle Mus-Veteau
Dr. Isabelle Mus-Veteau
E-Mail
Website
Guest Editor
Institute of Molecular and Cellular Pharmacology, French National Centre for Scientific Research, Valbonne, France
Interests: chemotherapy resistance; multidrug transporters; patched; inhibitors of drug efflux
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Many proteins or micro-RNAs involved in the development of cancers, proliferation, and apoptosis of cancer cells have been discovered, and many molecules have been developed against these targets to fight cancer. Despite these advances, too many patients still die of cancer due to late detection, low treatment efficacy, or resistance to treatment leading to tumor restart and/or metastasis. It is therefore necessary and urgent to pursue our efforts to find new targets and new molecules to improve the efficacy of cancer treatments and patient survival. This Special Issue will highlight present efforts in medicinal chemistry and pharmacology with studies of new molecules directed against known targets or against targets recently identified as being involved in the development and progression of cancer, and new molecules inhibitors of the resistance of cancer cells to current therapies, which is the main factor of treatment failure. This Special Issue welcomes original and review articles on recent advances and emerging concepts in the latest strategies in the fight against cancer.
You may choose our Joint Special Issue in Chemistry.
Dr. Isabelle Mus-Veteau
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molecules is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- anticancer/antitumor treatment
- novel anticancer molecules
- inhibitors of chemotherapy resistance
- chemotherapy
- targeted therapy
- medicinal chemistry
- pharmacology
Related Special Issue
Published Papers (12 papers)
Open AccessArticle
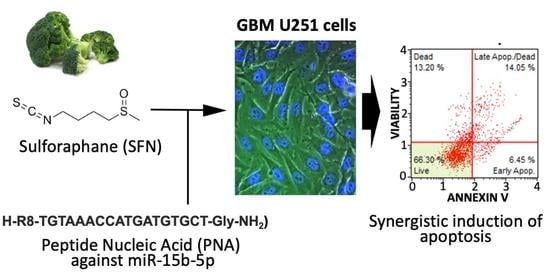
Treatment of Human Glioblastoma U251 Cells with Sulforaphane and a Peptide Nucleic Acid (PNA) Targeting miR-15b-5p: Synergistic Effects on Induction of Apoptosis
by
Jessica Gasparello, Chiara Papi, Matteo Zurlo, Laura Gambari, Andrea Rozzi, Alex Manicardi, Roberto Corradini, Roberto Gambari and Alessia Finotti
Cited by 17 | Viewed by 4824
Abstract
Glioblastoma multiforme (GBM) is a lethal malignant tumor accounting for 42% of the tumors of the central nervous system, the median survival being 15 months. At present, no curative treatment is available for GBM and new drugs and therapeutic protocols are urgently needed.
[...] Read more.
Glioblastoma multiforme (GBM) is a lethal malignant tumor accounting for 42% of the tumors of the central nervous system, the median survival being 15 months. At present, no curative treatment is available for GBM and new drugs and therapeutic protocols are urgently needed. In this context, combined therapy appears to be a very interesting approach. The isothiocyanate sulforaphane (SFN) has been previously shown to induce apoptosis and inhibit the growth and invasion of GBM cells. On the other hand, the microRNA miR-15b is involved in invasiveness and proliferation in GBM and its inhibition is associated with the induction of apoptosis. On the basis of these observations, the objective of the present study was to determine whether a combined treatment using SFN and a peptide nucleic acid interfering with miR-15b-5p (PNA-a15b) might be proposed for increasing the pro-apoptotic effects of the single agents. To verify this hypothesis, we have treated GMB U251 cells with SFN alone, PNA-a15b alone or their combination. The cell viability, apoptosis and combination index were, respectively, analyzed by calcein staining, annexin-V and caspase-3/7 assays, and RT-qPCR for genes involved in apoptosis. The efficacy of the PNA-a15b determined the miR-15b-5p content analyzed by RT-qPCR. The results obtained indicate that SFN and PNA-a15b synergistically act in inducing the apoptosis of U251 cells. Therefore, the PNA-a15b might be proposed in a “combo-therapy” associated with SFN. Overall, this study suggests the feasibility of using combined treatments based on PNAs targeting miRNA involved in GBM and nutraceuticals able to stimulate apoptosis.
Full article
►▼
Show Figures
Open AccessArticle
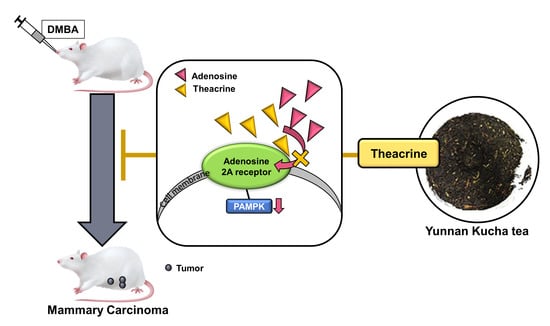
Attenuation of Tumor Development in Mammary Carcinoma Rats by Theacrine, an Antagonist of Adenosine 2A Receptor
by
Cian-Fen Jhuo, Yu-Yu Hsu, Wen-Ying Chen and Jason T. C. Tzen
Cited by 6 | Viewed by 3495
Abstract
Caffeine has been reported to induce anti-tumor immunity for attenuating breast cancer by blocking the adenosine 2A receptor. Molecular modeling showed that theacrine, a purine alkaloid structurally similar to caffeine, might be an antagonist of the adenosine 2A receptor equivalent to or more
[...] Read more.
Caffeine has been reported to induce anti-tumor immunity for attenuating breast cancer by blocking the adenosine 2A receptor. Molecular modeling showed that theacrine, a purine alkaloid structurally similar to caffeine, might be an antagonist of the adenosine 2A receptor equivalent to or more effective than caffeine. Theacrine was further demonstrated to be an effective antagonist of the adenosine 2A receptor as its concurrent supplementation significantly reduced the elevation of AMPK phosphorylation level in MCF-7 human breast cells induced by CGS21680, an agonist of adenosine 2A receptors. In an animal model, the development of mammary carcinoma induced by 7,12-Dimethylbenz[a]anthracene in Sprague–Dawley rats could be attenuated by daily supplement of theacrine of 50 or 100 mg/kg body weight. Both expression levels of cleaved-caspase-3/pro-caspase-3 and granzyme B in tumor tissues were significantly elevated when theacrine was supplemented, indicating the induction of programmed cell death in tumor cells might be involved in the attenuation of mammary carcinoma. Similar to the caffeine, significant elevation of interferon-γ and tumor necrosis factor-α was observed in the serum and tumor tissues of rats after the theacrine supplement of 50 mg/kg body weight. Taken together, theacrine is an effective antagonist of adenosine 2A receptors and possesses great potential to be used to attenuate breast cancer.
Full article
►▼
Show Figures
Open AccessArticle
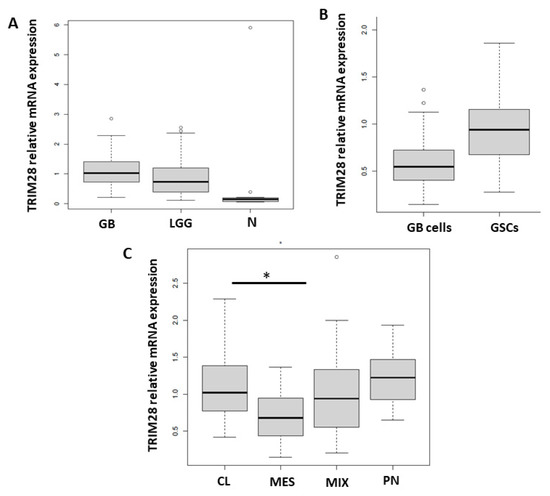
TRIM28 Selective Nanobody Reduces Glioblastoma Stem Cell Invasion
by
Andrej Porčnik, Metka Novak, Barbara Breznik, Bernarda Majc, Barbara Hrastar, Neja Šamec, Alja Zottel, Ivana Jovčevska, Miloš Vittori, Ana Rotter, Radovan Komel and Tamara Lah Turnšek
Cited by 22 | Viewed by 3694
Abstract
Glioblastoma (GB), is the most common and aggressive malignant primary brain tumour in adults. Intra- and inter-tumour heterogeneity, infiltrative GB cell invasion and presence of therapy-resistant GB stem cells (GSCs) represent major obstacles to favourable prognosis and poor therapy response. Identifying the biomarkers
[...] Read more.
Glioblastoma (GB), is the most common and aggressive malignant primary brain tumour in adults. Intra- and inter-tumour heterogeneity, infiltrative GB cell invasion and presence of therapy-resistant GB stem cells (GSCs) represent major obstacles to favourable prognosis and poor therapy response. Identifying the biomarkers of the most aggressive tumour cells and their more efficient targeting strategies are; therefore, crucial. Recently, transcription factor TRIM28 has been identified as a GB biomarker and, in this study, we have shown high expression of TRIM28 in GB and in low grade gliomas as well as higher expression in GSCs vs. differentiated GB cells, although in both cases not significant. We demonstrated significant in vitro inhibition of GB cells and GSCs invasiveness and spread in zebrafish brains in vivo by anti-TRIM28 selective nanobody NB237. TRIM28 was also enriched in GB (tumour) core and associated with the expression of stem cell genes, but was not prognostic for overall survival. However, based on the above results, we conclude that TRIM28 nanobody NB237 offers a new opportunity as a GB therapeutic tool.
Full article
►▼
Show Figures
Open AccessArticle
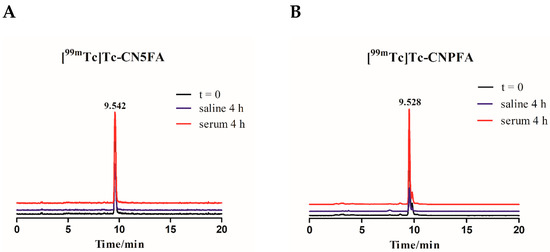
Preparation and Evaluation of Novel Folate Isonitrile 99mTc Complexes as Potential Tumor Imaging Agents to Target Folate Receptors
by
Junhong Feng, Xuran Zhang, Qing Ruan, Yuhao Jiang and Junbo Zhang
Cited by 10 | Viewed by 2453
Abstract
In order to seek novel technetium-99m folate receptor-targeting agents, two folate derivatives (CN5FA and CNPFA) were synthesized and radiolabeled to obtain [
99mTc]Tc-CN5FA and [
99mTc]Tc-CNPFA complexes, which exhibited high radiochemical purity (>95%) without purification, hydrophilicity, and good stability in vitro.
[...] Read more.
In order to seek novel technetium-99m folate receptor-targeting agents, two folate derivatives (CN5FA and CNPFA) were synthesized and radiolabeled to obtain [
99mTc]Tc-CN5FA and [
99mTc]Tc-CNPFA complexes, which exhibited high radiochemical purity (>95%) without purification, hydrophilicity, and good stability in vitro. The KB cell competitive binding experiments indicated that [
99mTc]Tc-CN5FA and [
99mTc]Tc-CNPFA had specificity to folate receptor. Biodistribution studies in KB tumor-bearing mice illustrated that [
99mTc]Tc-CN5FA and [
99mTc]Tc-CNPFA had specific tumor uptake. Compared with [
99mTc]Tc-CN5FA, the tumor/muscle ratios of [
99mTc]Tc-CNPFA were higher, resulting in a better SPECT/CT imaging background. According to the results, the two
99mTc complexes have potential as tumor imaging agents to target folate receptors.
Full article
►▼
Show Figures
Open AccessArticle
Fecal Metabolic Profiling of Breast Cancer Patients during Neoadjuvant Chemotherapy Reveals Potential Biomarkers
by
Oumaima Zidi, Nessrine Souai, Henda Raies, Farhat Ben Ayed, Amel Mezlini, Sonia Mezrioui, Fabrice Tranchida, Jean-Marc Sabatier, Amor Mosbah, Ameur Cherif, Laetitia Shintu and Soumaya Kouidhi
Cited by 20 | Viewed by 5978
Abstract
Breast cancer (BC) is the most common form of cancer among women worldwide. Despite the huge advancements in its treatment, the exact etiology of breast cancer still remains unresolved. There is an increasing interest in the role of the gut microbiome in modulating
[...] Read more.
Breast cancer (BC) is the most common form of cancer among women worldwide. Despite the huge advancements in its treatment, the exact etiology of breast cancer still remains unresolved. There is an increasing interest in the role of the gut microbiome in modulating the anti-cancer therapeutic response. It seems that alteration of the microbiome-derived metabolome potentially promotes carcinogenesis. Taken together, metabolomics has arisen as a fascinating new omics field to screen promising metabolic biomarkers. In this study, fecal metabolite profiling was performed using NMR spectroscopy, to identify potential biomarker candidates that can predict response to neoadjuvant chemotherapy (NAC) for breast cancer. Metabolic profiles of feces from patients (
n = 8) following chemotherapy treatment cycles were studied. Interestingly, amino acids were found to be upregulated, while lactate and fumaric acid were downregulated in patients under the second and third cycles compared with patients before treatment. Furthermore, short-chain fatty acids (SCFAs) were significantly differentiated between the studied groups. These results strongly suggest that chemotherapy treatment plays a key role in modulating the fecal metabolomic profile of BC patients. In conclusion, we demonstrate the feasibility of identifying specific fecal metabolic profiles reflecting biochemical changes that occur during the chemotherapy treatment. These data give an interesting insight that may complement and improve clinical tools for BC monitoring.
Full article
►▼
Show Figures
Open AccessArticle
Methiothepin Increases Chemotherapy Efficacy against Resistant Melanoma Cells
by
Nelly Durand, Méliné Simsir, Laurie Signetti, Fabien Labbal, Robert Ballotti and Isabelle Mus-Veteau
Cited by 10 | Viewed by 2817
Abstract
We previously reported that methiothepin, a small molecule known as a nonselective serotonin 5-HT receptor antagonist, inhibited the doxorubicin efflux activity of the Hedgehog receptor Ptch1 and enhanced the cytotoxic, pro-apoptotic, anti-proliferative, and anti-clonogenic effects of doxorubicin on adrenocortical carcinoma cells. Here, we
[...] Read more.
We previously reported that methiothepin, a small molecule known as a nonselective serotonin 5-HT receptor antagonist, inhibited the doxorubicin efflux activity of the Hedgehog receptor Ptch1 and enhanced the cytotoxic, pro-apoptotic, anti-proliferative, and anti-clonogenic effects of doxorubicin on adrenocortical carcinoma cells. Here, we show that methiothepin also inhibits doxorubicin efflux and increases doxorubicin cytotoxicity in melanoma cells which endogenously overexpress Ptch1. Melanoma patients having the BRAF
V600E mutation are treated with vemurafenib, an inhibitor of BRAF
V600E, often in combination with trametinib, an inhibitor of MEK. Almost all patients ultimately acquire resistance to the treatment leading to disease progression. Here, we report that methiothepin overcomes the resistance of BRAF
V600E melanoma cells by enhancing the cytotoxicity of vemurafenib and trametinib on these cells leading to melanoma cells death. We observe that the addition of methiothepin to vemurafenib prevents migration of resistant melanoma cells more efficiently than vemurafenib alone. Our results provide an additional proof that Ptch1 drug efflux inhibition increases the effectiveness of anti-cancer treatments and overcomes resistance of melanoma cells expressing Ptch1.
Full article
►▼
Show Figures
Open AccessArticle
Expression of Luteinizing Hormone-Releasing Hormone (LHRH) and Type-I LHRH Receptor in Transitional Cell Carcinoma Type of Human Bladder Cancer
by
Zsuzsanna Szabó, Balázs Dezső, Klára Fodor, Krisztián Szegedi, Tibor Flaskó, Erzsébet Szabó, Gábor Oláh, Éva Sipos, Nikoletta Dobos, János Gardi, Andrew V. Schally and Gábor Halmos
Cited by 3 | Viewed by 3321
Abstract
Bladder cancer (BC) is the tenth most frequently detected cancer in both sexes. Type-I luteinizing hormone-releasing hormone (LHRH) receptor (LHRH-R-I) is expressed not only in the pituitary, but also in several types of cancer disease. There are few data about LHRH-R-I expression in
[...] Read more.
Bladder cancer (BC) is the tenth most frequently detected cancer in both sexes. Type-I luteinizing hormone-releasing hormone (LHRH) receptor (LHRH-R-I) is expressed not only in the pituitary, but also in several types of cancer disease. There are few data about LHRH-R-I expression in human BC. This study aimed to investigate the expression of LHRH and LHRH-R-I in the transitional cell carcinoma (TCC) type of human BC. RNA was extracted from 24 human bladder tumor specimens and three BC cell lines. RT-PCR was performed to detect mRNA for LHRH and LHRH-R-I. The protein of LHRH-R-I was further studied by immunohistochemistry (IHC), ligand competition assay, and Western Blot. PCR products of LHRH were found in 19 of 24 (79%) specimens and mRNA of LHRH-R-I was detected in 20 of 24 specimens (83%). Positive immunostaining for LHRH-R-I with different expression intensity was found in all samples examined, showing negative correlation with TCC grade. Radioligand binding studies also showed the presence of specific LHRH-R-I and high affinity binding of LHRH analogs. The high incidence of LHRH-R in BC suggests that it could serve as a molecular target for therapy of human BC with cytotoxic LHRH analogs or modern powerful antagonistic analogs of LHRH.
Full article
►▼
Show Figures
Open AccessReview
Unlocking the Secrets of Cancer Stem Cells with γ-Secretase Inhibitors: A Novel Anticancer Strategy
by
Maryam Ghanbari-Movahed, Zahra Ghanbari-Movahed, Saeideh Momtaz, Kaitlyn L. Kilpatrick, Mohammad Hosein Farzaei and Anupam Bishayee
Cited by 16 | Viewed by 4191
Abstract
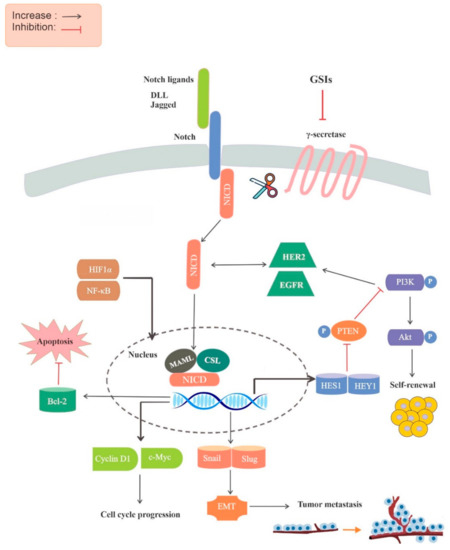
The dysregulation of Notch signaling is associated with a wide variety of different human cancers. Notch signaling activation mostly relies on the activity of the γ-secretase enzyme that cleaves the Notch receptors and releases the active intracellular domain. It is well-documented that γ-secretase
[...] Read more.
The dysregulation of Notch signaling is associated with a wide variety of different human cancers. Notch signaling activation mostly relies on the activity of the γ-secretase enzyme that cleaves the Notch receptors and releases the active intracellular domain. It is well-documented that γ-secretase inhibitors (GSIs) block the Notch activity, mainly by inhibiting the oncogenic activity of this pathway. To date, several GSIs have been introduced clinically for the treatment of various diseases, such as Alzheimer’s disease and various cancers, and their impacts on Notch inhibition have been found to be promising. Therefore, GSIs are of great interest for cancer therapy. The objective of this review is to provide a systematic review of in vitro and in vivo studies for investigating the effect of GSIs on various cancer stem cells (CSCs), mainly by modulation of the Notch signaling pathway. Various scholarly electronic databases were searched and relevant studies published in the English language were collected up to February 2020. Herein, we conclude that GSIs can be potential candidates for CSC-targeting therapy. The outcome of our study also indicates that GSIs in combination with anticancer drugs have a greater inhibitory effect on CSCs.
Full article
►▼
Show Figures
Open AccessArticle
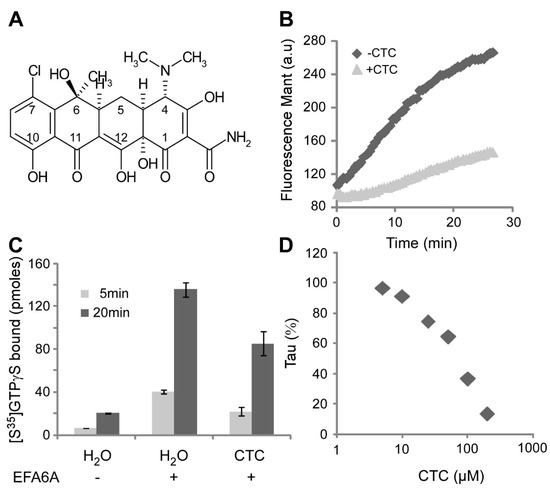
Chlortetracycline, a Novel Arf Inhibitor That Decreases the Arf6-Dependent Invasive Properties of Breast Cancer Cells
by
Eric Macia, Monserrat Vazquez-Rojas, Alessia Robiolo, Racha Fayad, Sophie Abélanet, Isabelle Mus-Veteau, Fabien Fontaine-Vive, Mohamed Mehiri, Frédéric Luton and Michel Franco
Cited by 8 | Viewed by 3332
Abstract
Breast cancer is a major disease for women worldwide, where mortality is associated with tumour cell dissemination to distant organs. While the number of efficient anticancer therapies increased in the past 20 years, treatments targeting the invasive properties of metastatic tumour cells are
[...] Read more.
Breast cancer is a major disease for women worldwide, where mortality is associated with tumour cell dissemination to distant organs. While the number of efficient anticancer therapies increased in the past 20 years, treatments targeting the invasive properties of metastatic tumour cells are still awaited. Various studies analysing invasive breast cancer cell lines have demonstrated that Arf6 is an important player of the migratory and invasive processes. These observations make Arf6 and its regulators potential therapeutic targets. As of today, no drug effective against Arf6 has been identified, with one explanation being that the activation of Arf6 is dependent on the presence of lipid membranes that are rarely included in drug screening. To overcome this issue we have set up a fluorescence-based high throughput screening that follows overtime the activation of Arf6 at the surface of lipid membranes. Using this unique screening assay, we isolated several compounds that affect Arf6 activation, among which the antibiotic chlortetracycline (CTC) appeared to be the most promising. In this report, we describe CTC in vitro biochemical characterization and show that it blocks both the Arf6-stimulated collective migration and cell invasion in a 3D collagen I gel of the invasive breast cancer cell line MDA-MB-231. Thus, CTC appears as a promising hit to target deadly metastatic dissemination and a powerful tool to unravel the molecular mechanisms of Arf6-mediated invasive processes.
Full article
►▼
Show Figures
Open AccessArticle
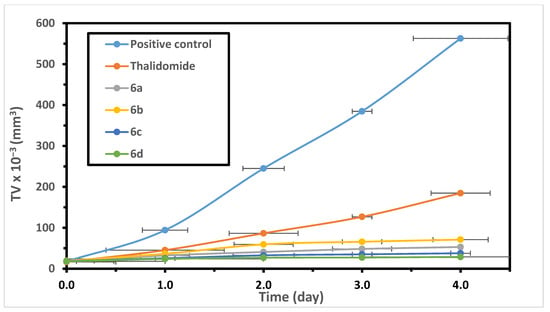
In Vitro and In Vivo Antitumor Activity of Indolo[2,3-b] Quinolines, Natural Product Analogs from Neocryptolepine Alkaloid
by
Najla Altwaijry, Samah El-Ghlban, Ibrahim E.-T. El Sayed, Mohamed El-Bahnsawye, Asmaa I. Bayomi, Rehab M. Samaka, Elkhabiry Shaban, Elshaymaa I. Elmongy, Thanaa A. El-Masry, Hytham M. A. Ahmed and Nashwah G. M. Attallah
Cited by 21 | Viewed by 4688
Abstract
Neocryptolepine (5-methyl-5H-indolo[2,3-b] quinoline) analogs were synthesized and evaluated in vitro and in vivo for their effect versus Ehrlich ascites carcinoma (EAC). The analogs showed stronger cytotoxic activity against EAC cells than the reference drug. The in vivo evaluation of the target compounds against
[...] Read more.
Neocryptolepine (5-methyl-5H-indolo[2,3-b] quinoline) analogs were synthesized and evaluated in vitro and in vivo for their effect versus Ehrlich ascites carcinoma (EAC). The analogs showed stronger cytotoxic activity against EAC cells than the reference drug. The in vivo evaluation of the target compounds against EAC-induced solid tumor in the female albino Swiss mice revealed a remarkable decrease in the tumor volume (TV) and hepatic lipid peroxidation. A noticeable increase of both superoxide dismutase (SOD) and catalase (CAT) levels was reported (
p < 0.001), which set-forth proof of their antioxidant effect. In addition, the in vitro antioxidant activity of the neocryptolepine analogs was screened out using the DPPH method and showed promising activities activity. The histopathological investigations affirmed that the tested analogs have a remarkable curative effect on solid tumors with minimal side-effect on the liver. The study also includes illustrated mechanism of the antitumor activity at the cell level by flow cytometry. The cell cycle analysis showed that the neocryptolepine analogs extensively increase the aggregation of tumor cells in three phases of the cell cycle (G0/G1, S and G2/M) with the emergence of a hypo-diploid DNA content peak (sub-G1) in the cell cycle experiments, which is a clear-cut for the apoptotic cell population. Furthermore, the immunological study manifested a significant elevation in splenic lymphocyte count (
p < 0.001) with the elevation of the responsiveness of lymphocytes to phytohemagglutinin (PHA). These results indicate that these naturally-based neocryptolepine alkaloids exhibit marked antitumor activity in vivo and represent an important lead in the development of natural-based anticancer drugs.
Full article
►▼
Show Figures
Open AccessArticle
N-phenyl-6-chloro-4-hydroxy-2-quinolone-3-carboxamides: Molecular Docking, Synthesis, and Biological Investigation as Anticancer Agents
by
Dima A. Sabbah, Rawan A. Haroon, Sanaa K. Bardaweel, Rima Hajjo and Kamal Sweidan
Cited by 13 | Viewed by 5157
Abstract
Cancer is a multifactorial disease and the second leading cause of death worldwide. Diverse factors induce carcinogenesis, such as diet, smoking, radiation, and genetic defects. The phosphatidylinositol 3-kinase (PI3Kα) has emerged as an attractive target for anticancer drug design. Eighteen derivatives of
N
[...] Read more.
Cancer is a multifactorial disease and the second leading cause of death worldwide. Diverse factors induce carcinogenesis, such as diet, smoking, radiation, and genetic defects. The phosphatidylinositol 3-kinase (PI3Kα) has emerged as an attractive target for anticancer drug design. Eighteen derivatives of
N-phenyl-6-chloro-4-hydroxy-2-quinolone-3-carboxamide were synthesized and characterized using FT-IR, NMR (
1H and
13C), and high-resolution mass spectra (HRMS). The series exhibited distinct antiproliferative activity (IC
50 µM) against human epithelial colorectal adenocarcinoma (Caco-2) and colon carcinoma (HCT-116) cell lines, respectively: compounds
16 (37.4, 8.9 µM),
18 (50.9, 3.3 µM),
19 (17.0, 5.3 µM), and
21 (18.9, 4.9 µM). The induced-fit docking (IFD) studies against PI3Kαs showed that the derivatives occupy the PI3Kα binding site and engage with key binding residues.
Full article
►▼
Show Figures
Open AccessArticle
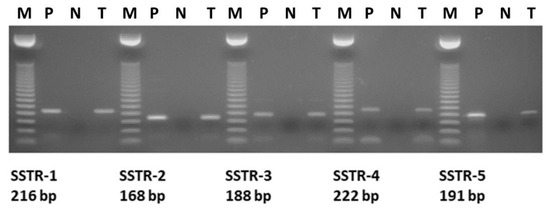
Expression of Somatostatin Receptor Subtypes (SSTR-1–SSTR-5) in Pediatric Hematological and Oncological Disorders
by
Kristof Harda, Zsuzsanna Szabo, Eva Juhasz, Balazs Dezso, Csongor Kiss, Andrew V. Schally and Gabor Halmos
Cited by 7 | Viewed by 4501
Abstract
Hematological and oncological disorders represent leading causes of childhood mortality. Neuropeptide somatostatin (SST) has been previously demonstrated in various pediatric tumors, but limited information exists on the expression and characteristics of SST receptors (SSTR) in hematological and oncological disorders of children. We aimed
[...] Read more.
Hematological and oncological disorders represent leading causes of childhood mortality. Neuropeptide somatostatin (SST) has been previously demonstrated in various pediatric tumors, but limited information exists on the expression and characteristics of SST receptors (SSTR) in hematological and oncological disorders of children. We aimed to investigate the expression of mRNA for SSTR subtypes (SSTR-1–5) in 15 pediatric hematological/oncological specimens by RT-PCR. The presence and binding characteristics of SSTRs were further studies by ligand competition assay. Our results show that the pediatric tumor samples highly expressed mRNA for the five SSTR subtypes with various patterns. The mRNA for SSTR-2 was detected in all specimens independently of their histological type. A Hodgkin lymphoma sample co-expressed mRNA for all five SSTR subtypes. SSTR-3 and SSTR-5 were detected only in malignant specimens, such as rhabdomyosarcoma, Hodgkin lymphoma, acute lymphoblastic leukemia, and a single nonmalignant condition, hereditary spherocytosis. The incidence of SSTR-1 and SSTR-4 was similar (60%) in the 15 specimens investigated. Radioligand binding studies demonstrated the presence of specific SSTRs and high affinity binding of SST analogs in pediatric solid tumors investigated. The high incidence of SSTRs in hematological and oncological disorders in children supports the merit of further investigation of SSTRs as molecular targets for diagnosis and therapy.
Full article
►▼
Show Figures