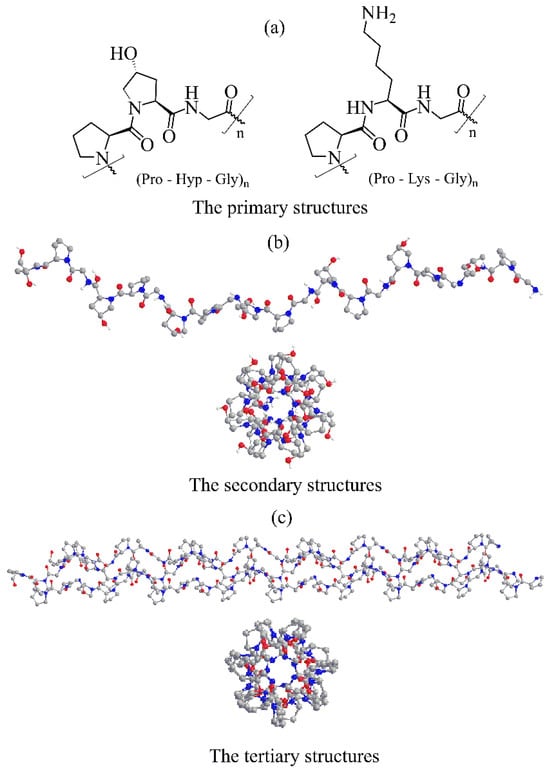
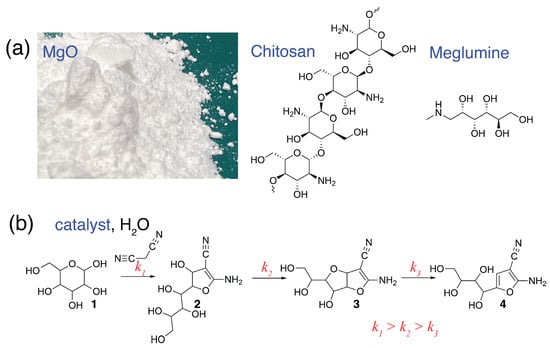
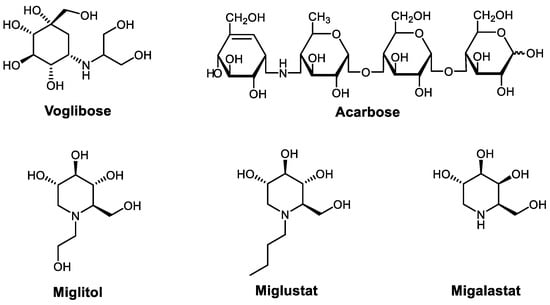
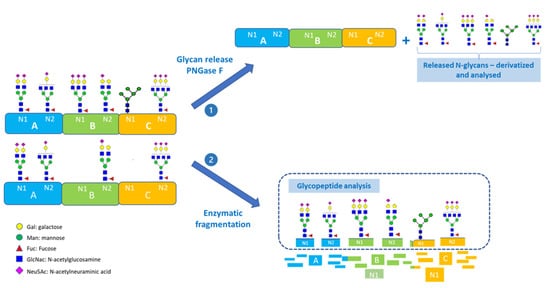
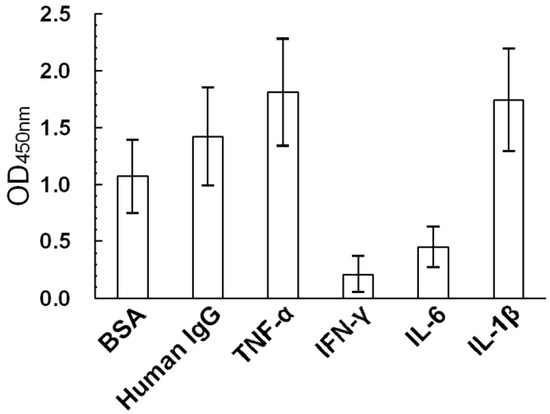
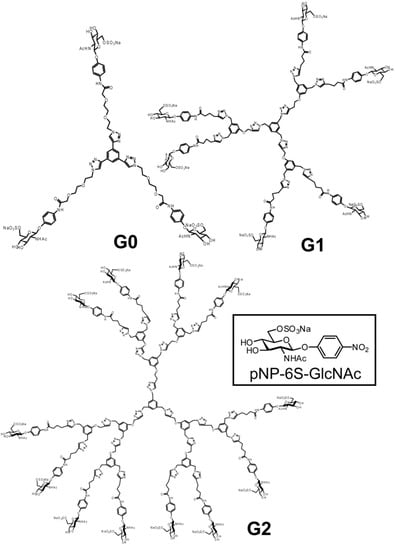
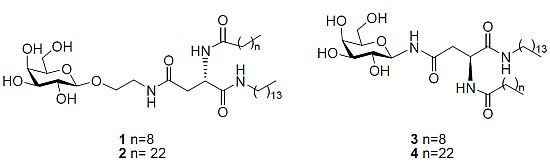
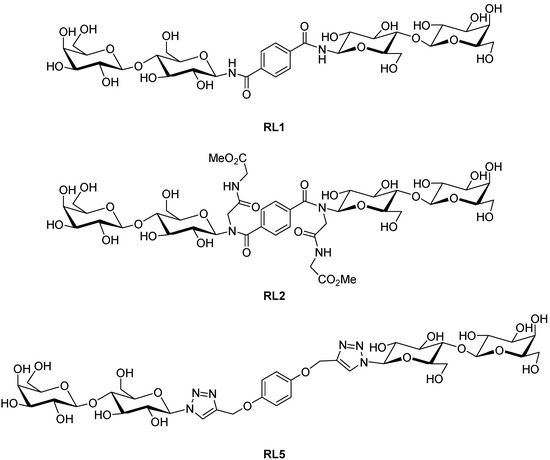
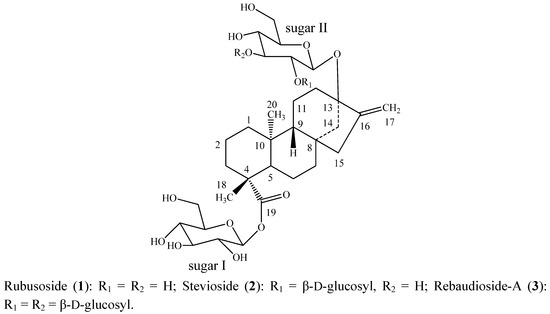
Advances in Glycosciences
A topical collection in Molecules (ISSN 1420-3049). This collection belongs to the section "Chemical Biology".
Viewed by 188164Editors
Interests: carbohydrate chemistry; molecular recognition; cyclodextrins; supramolecular self-assembling systems; drug and gene delivery
Interests: carbohydrate chemistry; glycoconjugates; glycopeptides; glycolipids; glycomimetics; molecular scaffolds
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
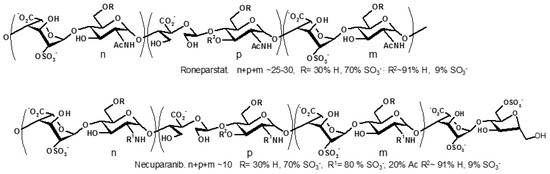
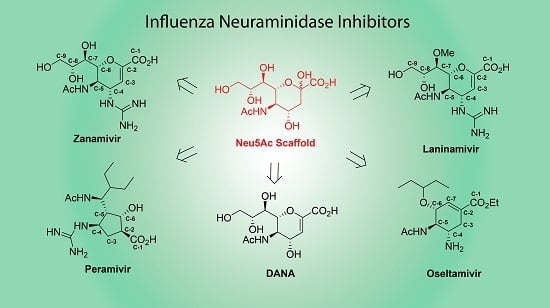
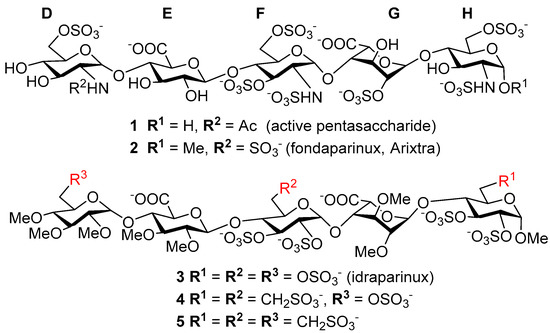
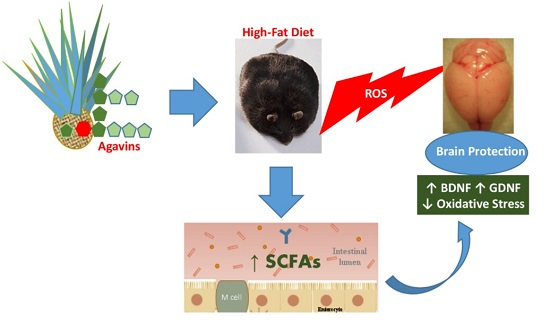
Advancements in synthetic methods, molecular biology and analytical techniques have evidenced the key roles that carbohydrates and their conjugates (from simple saccharide units to complex glycans) play in biological processes, such as protein folding, infection, cancer and immune recognition. Despite of the significant achievements in recent years, a number of challenges still remain to be addressed in the field of Glycoscience. Carbohydrate chemists can contribute to the development of robust methodologies for the preparation of synthetic glycans, glycomimetics and glycoconjugates. Carbohydrate-based sensors can serve as tools to interrogate the intricate mechanisms of carbohydrate recognition in biological environments. Hierarchical control over glycan display in macromolecular scaffolds might furnish nanometric probes to interfere biological processes with tailored purposes. New analytical techniques can lead to glycan profiling for identification of disease biomarkers. Research in Glycoscience can lead to breakthroughs in the development of new therapeutics, vaccines, diagnostic and delivery tools, among other biomedical applications.
The Topical Collection “Advances in Glycosciences” aims to highlight the breadth of topics of interest for the scientific community in the Glycosciences and provide some recent examples of significant advances in the field.
We invite you to submit research articles and comprehensive reviews related to the above topics to this Topical Collection, part of the journal Molecules. Contributions addressing research in Glycosicence and carbohydrate chemistry in general, will also be suitable for the scope of publication in this collection.
Dr. Juan M. Benito
Dr. Trinidad Velasco-Torrijos
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molecules is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- glycoconjugates
- carbohydrate chemistry
- glycodrugs
- glycoprobes
- glyconanomaterials
- carbohydrate recognition
Related Special Issue
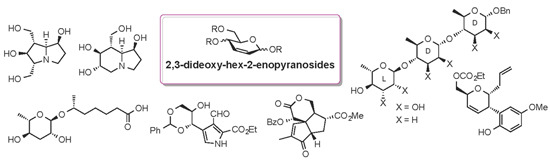
- Advances in Carbohydrate Chemistry 2012 in Molecules (15 articles - displayed below)