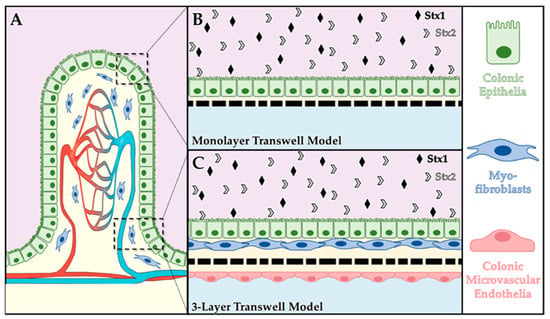
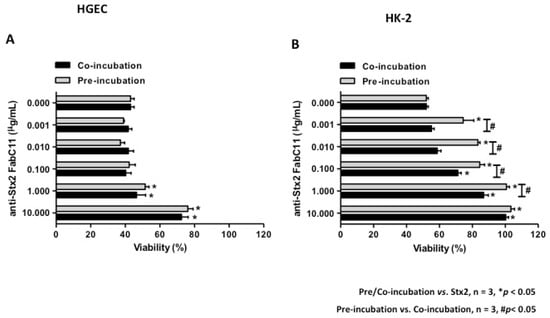
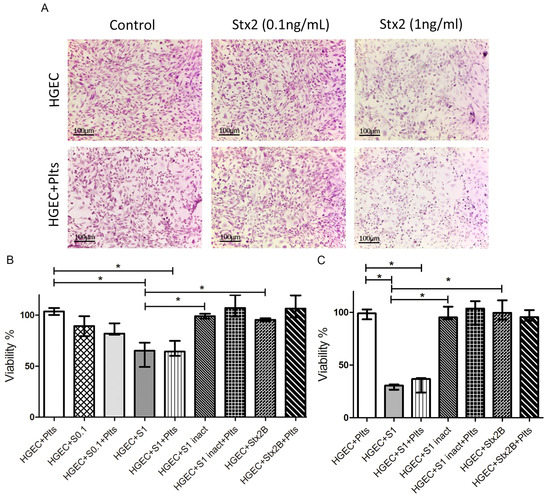
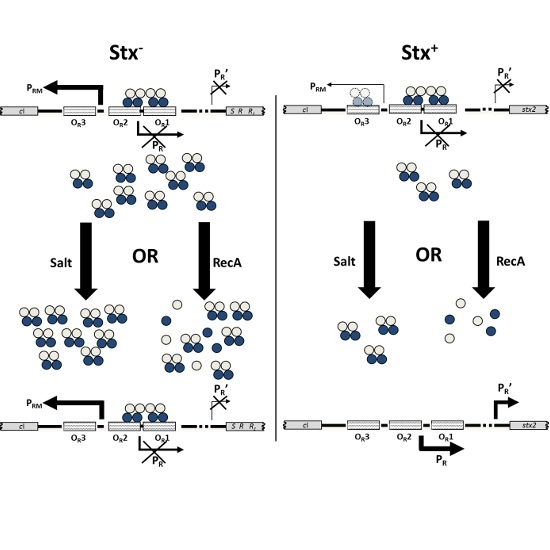
Shiga Toxins
A topical collection in Toxins (ISSN 2072-6651). This collection belongs to the section "Bacterial Toxins".
Viewed by 104867Editors
Interests: Evolution, distribution and role of exotoxin-encoding bacteria and phages in the environment; Identification and characterization of bacterial anti-predator defense mechanisms; Biotic and abiotic factors that govern the stability lambdoid prophages; DNA structure effects on protein-DNA Interactions
Special Issues, Collections and Topics in MDPI journals
Interests: glycosphingolipids(GSLs); GSL metabolism; GSL traffic; GSL function; shiga toxin; verotoxin cholera toxin; globotriaosylceramide; glucosylceramide; endoplasmic reticulum associated degradation (ERAD)
Special Issues, Collections and Topics in MDPI journals
Interests: Factors governing STEC in the environment; Biotic and abiotic factors influencing water quality; Bacterial and viral distribution in freshwater; The emergence of harmful algal blooms in aquatic environments
Topical Collection Information
Dear Colleagues,
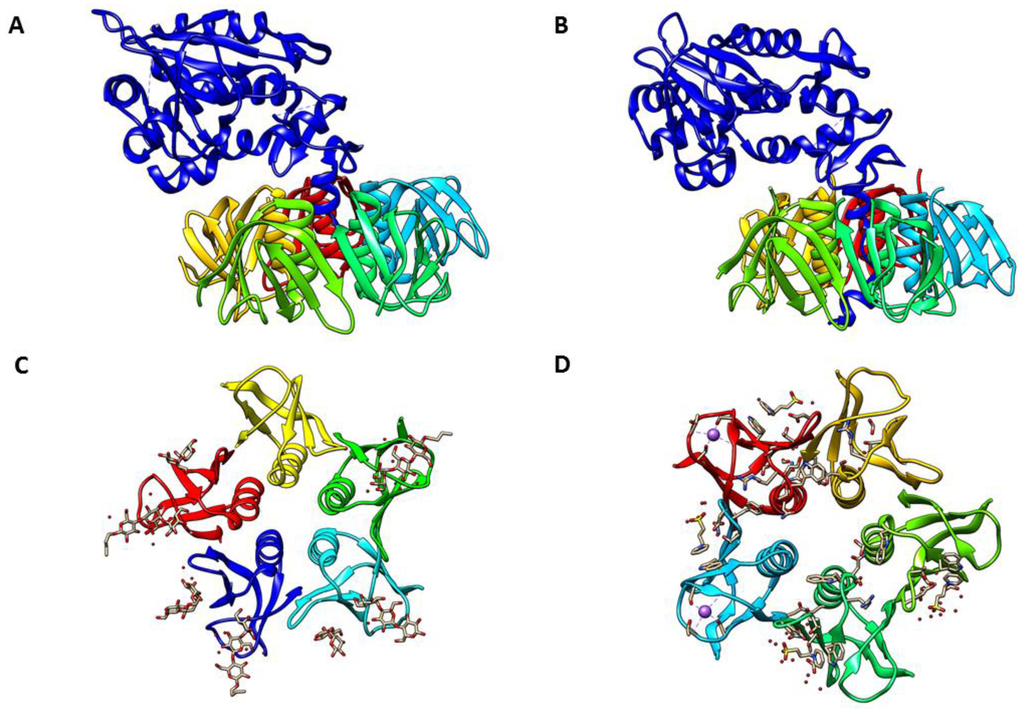
Shiga toxins are the main virulence factor of a group of Shiga toxin-encoding E. coli (STEC) strains that cause severe human diseases, such as hemorrhagic colitis and hemolytic uremic syndrome. Shiga toxin is implicated in over 10,000 cases of human illness annually in the United States alone, and infection with STEC carries a mortality rate as high as 10%. Shiga toxin intoxication is the number one cause of acute renal failure in children. Alarmingly, the incidence of Shiga toxin-related illness is increasing; in the two decades following its emergence, outbreaks of disease caused by STEC increased >20-fold. In addition to contaminated food, STEC outbreaks are increasingly associated with environmental contamination of water. Therefore, factors that impact the environmental survival of STEC clearly have a role in regulating the transmission of these pathogens to humans.
The Shiga toxin family is divided into several toxin subtypes, each of which is apparently differentially involved in causing human disease. Shiga toxins are encoded by a family of bacteriophage that are related to the well-characterized nontoxic coliphage λ. A recent expansion in the numbers of available STEC genome sequences and Shiga toxin encoding phages indicate that both the families of Stx-encoding phage and the bacteria that harbor them are large and display a much higher diversity than previously thought. Phage diversity may play an important role in the dissemination of Shiga-toxin genes and the emergence of new STEC strains, but also in the regulation of Shiga toxin production.
Prof. Dr. Gerald B. Koudelka
Dr. Steven A Mauro
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a double-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Toxins is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
"A similar collection Shiga Toxins can be found at https://www.mdpi.com/journal/toxins/special_issues/shiga
Keywords
- Shiga toxin
- Bacteria
- Bacteriophage
- STEC
- E. coli O157
- Virulence
- Diversity
Related Special Issue
- Shiga Toxin in Toxins (6 articles - displayed below)