Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering
Abstract
:1. Introduction
2. Experiment Details
3. Results and Discussion
3.1. Chemical Composition
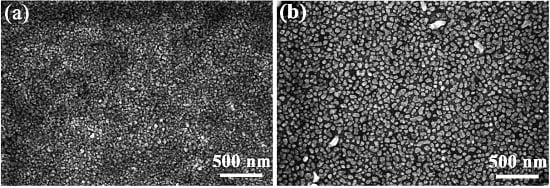
3.2. Structure and Thermal Stability
3.3. Mechanical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principle elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Gan, J.Y.; Lin, S.J.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35A, 2533–2536. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high entropy alloys. Ann. Chim. 2006, 31, 633–648. [Google Scholar]
- Zhang, Y.; Qiao, J.W.; Liaw, P.K. A brief review of high entropy alloys and serration behavior and flow units. J. Iron Steel Res. Int. 2016, 23, 2–6. [Google Scholar] [CrossRef]
- Li, J.M.; Yang, X.; Zhu, R.L.; Zhang, Y. Corrosion and serration behaviors of TiZr0.5NbCr0.5VxMoy high entropy alloys in aqueous environments. Metals 2014, 4, 597–608. [Google Scholar] [CrossRef]
- Chang, Z.C.; Liang, S.C.; Han, S.; Chen, Y.K.; Shieu, F.S. Characteristics of TiVCrAlZr multi-element nitride films prepared by reactive sputtering. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 2504–2509. [Google Scholar] [CrossRef]
- Liang, S.C.; Chang, Z.C.; Tsai, D.C.; Lin, Y.C.; Sung, H.S.; Deng, M.J.; Shieu, F.S. Structure and Mechanical Properties of Multi-element (TiVCrZrHf)N Coatings by Reactive Magnetron Sputtering. Appl. Surf. Sci. 2011, 258, 399–403. [Google Scholar] [CrossRef]
- Tsai, D.C.; Chang, Z.C.; Kuo, B.H.; Chang, S.Y.; Shieu, F.S. Effects of silicon content on the structure and properties of (AlCrMoTaTi)N coatings by reactive magnetron sputtering. J. Alloys Comp. 2014, 616, 646–651. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.B.; Hou, C.; Li, J.C.; Jiang, Q. Dense and smooth amorphous films of multicomponent FeCoNiCuVZrAl high-entropy alloy deposited by direct current magnetron sputtering. Mater. Des. 2013, 46, 675–679. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Shi, L.; Cai, B.; Wang, M.X. Structure and properties of (AlCrMnMoNiZrB0.1)Nx coatings prepared by reactive DC sputtering. Appl. Surf. Sci. 2011, 257, 7172–7178. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhan, Y.Z.; Zhang, G.H.; She, J.; Li, C.H. Effect of rare earth Y addition on the microstructure and mechanical properties of high entropy AlCoCrCuNiTi alloys. Mater. Des. 2010, 31, 1599–1602. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.Z.; Shen, J.Y.; Vilar, R. Thermal stability and oxidation resistance of laser clad TiVCrAlSi high entropy alloy coatings on Ti–6Al–4V alloy. Surf. Coat. Technol. 2011, 206, 1389–1395. [Google Scholar] [CrossRef]
- Tsai, M.H.; Wang, C.W.; Lai, C.H.; Yeh, J.W.; Gan, J.Y. Thermally stable amorphous (AlMoNbSiTaTiVZr)50N50nitride film as diffusion barrier in copper metallization. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Chang, S.Y.; Chen, D.S. Ultrathin (AlCrTaTiZr)Nx/AlCrTaTiZr Bilayer Structures with High Diffusion Resistance for Cu Interconnects. J. Electrochem. Soc. 2010, 157, G154–G159. [Google Scholar] [CrossRef]
- Liang, S.C.; Tsai, D.C.; Chang, Z.C.; Lin, T.N.; Shiao, M.H.; Shieu, F.S. Thermally Stable TiVCrZrHf Nitride Films as Diffusion Barriers in Copper Metallization. Electrochem. Solid State Lett. 2012, 15, H5–H8. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Films 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Sheng, W.J.; Yang, X.; Zhu, J.; Wang, C.; Zhang, Y. Amorphous phase stability of NbTiAlSiNx high-entropy films. Rare Metals 2016. accepted. [Google Scholar]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials; Wiley: New York, NY, USA, 1954. [Google Scholar]
- Tsai, D.C.; Chang, Z.C.; Kuo, L.Y.; Lin, T.J.; Lin, T.N. Oxidation resistance and structural evolution of (TiVCrZrHf)N coatings. Thin Solid Films 2013, 544, 580–587. [Google Scholar] [CrossRef]
- Wu, Z.F.; Wang, X.D.; Cao, Q.P.; Zhao, G.H.; Li, J.X.; Zhang, D.X.; Zhu, J.J.; Jiang, J.Z. Microstructure characterization of AlxCo1Cr1Cu1Fe1Ni1 (x = 0 and 2.5) high-entropy alloy films. J. Alloys Comp. 2014, 609, 137–142. [Google Scholar] [CrossRef]
- Tsai, C.W.; Lai, S.W.; Cheng, K.H.; Tsai, M.H.; Davison, A.; Tsau, C.H.; Yeh, J.W. Strong amorphization of high-entropy AlBCrSiTi nitride film. Thin Solid Films 2012, 520, 2613–2618. [Google Scholar] [CrossRef]
- Feng, X.G.; Tang, G.Z.; Gu, L.; Ma, X.X.; Sun, M.R.; Wang, L.Q. Preparation and characterization of TaNbTiW multi-element alloy films. Appl. Surf. Sci. 2012, 261, 447–453. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.Z. Effects of Annealing on the Microstructure and Properties of 6FeNiCoCrAlTiSi High-Entropy Alloy Coating Prepared by Laser Cladding. J. Thermal Spray Technol. 2011, 20, 1049–1055. [Google Scholar] [CrossRef]





| Element (at.%) | Nb | Ti | Al | Si | W |
|---|---|---|---|---|---|
| Nominal (target) | 20 | 20 | 20 | 20 | 20 |
| Actual (film) (deviation = ±0.5%) | 19.8 | 21.1 | 20.2 | 20.5 | 18.4 |
| Nb | Ti | Al | W | NbN | TiN | AlN | |
|---|---|---|---|---|---|---|---|
| Lattice constant () | 3.371 | a = 2.950; c = 4.686 | 4.050 | 3.158 | 4.40 | 4.24 | 4.04 |
| Atomic radius () [21] | 1.429 | 1.462 | 1.432 | 1.3367 | - | - | - |
| Structure | BCC | HCP | FCC | BCC | FCC | FCC | HCP |
| Composition | Method | Duration (°C) | Holding Time | Reference |
|---|---|---|---|---|
| (TiVCrZrHf)N | sputtering | 300 | 2 h in air | [23] |
| AlxCo1Cr1Cu1Fe1Ni1 | sputtering | 600 | 20 min vacuum | [24] |
| (AlBCrSiTi)N | sputtering | 700 | 2 h vacuum | [25] |
| TaNbTiW | sputtering | 700 | 90 min vacuum | [26] |
| 6FeNiCoCrAlTiSi | laser cladding | 750 | 5 h vacuum | [27] |
| (NbTiAlSiW)Nx | sputtering | 700 | 24 h vacuum | this work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, W.; Yang, X.; Wang, C.; Zhang, Y. Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering. Entropy 2016, 18, 226. https://doi.org/10.3390/e18060226
Sheng W, Yang X, Wang C, Zhang Y. Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering. Entropy. 2016; 18(6):226. https://doi.org/10.3390/e18060226
Chicago/Turabian StyleSheng, Wenjie, Xiao Yang, Cong Wang, and Yong Zhang. 2016. "Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering" Entropy 18, no. 6: 226. https://doi.org/10.3390/e18060226
APA StyleSheng, W., Yang, X., Wang, C., & Zhang, Y. (2016). Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering. Entropy, 18(6), 226. https://doi.org/10.3390/e18060226