How the Brain Becomes the Mind: Can Thermodynamics Explain the Emergence and Nature of Emotions?
Abstract
:1. Introduction
2. The Temporal Mind
- (1)
- Frequency dependence
- (2)
- Temporal organization
- (3)
- Associative model
3. Considerations of Entropy
3.1. Similarities of the Brain and Physical Systems Based on Entropy
3.2. Entropic Differences between the Brain and Physical Systems
4. Thermodynamic Regulation of the Neural System
- A stimulus triggers activation, which represents momentum and direction.
- Entropy-maximizing influences continuously adjust the large-scale spatial synchronization of oscillatory activity.
- The relaxation that recovers the resting state prepares the system for reactivation.
- Every cycle changes the brain’s synaptic balance and organization.
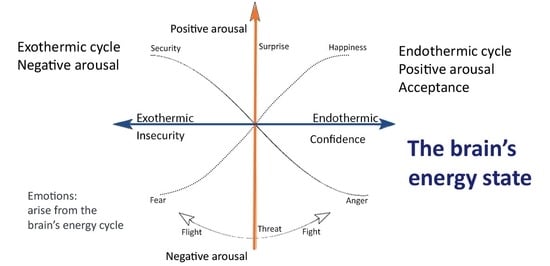
4.1. The Thermodynamics of Emotions
Emotions as the Fundamental Forces of Motivation
4.2. The Endothermic Cycle
4.3. The Exothermic Cycle
- However, in contrast to physical processes, the reversible brain activations stabilize low entropy, creating long-term adverse emotional and psychological outcomes.
- Long-term potentiation reduces the degrees of freedom due to the loss of synaptic complexity, producing repetitious, monotone thinking [122]. The negative thought pattern becomes more powerful and pessimistic through a Bayesian process, affecting behavior. For example, the severity of cognitive impairment in depression correlates with brain entropy reduction [122].
5. Spontaneous Processes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michel, M.; Fleming, S.; Lau, H.; Lee, A.; Martinez-Conde, S.; Passingham, R.; Peters, M.; Rahnev, D.; Sergent, C.; Liu, K. An Informal Internet Survey on the Current State of Consciousness Science. Front. Psychol. 2018, 9, 2134. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Beck, D.; Block, N.; Blumenfeld, H.; Brown, R.; Carmel, D.; Carrasco, M.; Chirimuuta, M.; Chun, M.; Cleeremans, A.; et al. Opportunities and challenges for a maturing science of consciousness. Nat. Hum. Behav. 2019, 3, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.C.; van Ede, F. Under the Mind’s Hood: What We Have Learned by Watching the Brain at Work. J. Neurosci. 2020, 40, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laeng, B.; Nabil, S.; Kitaoka, A. The Eye Pupil Adjusts to Illusorily Expanding Holes. Front. Hum. Neurosci. 2022, 11, 308. [Google Scholar] [CrossRef]
- Goldenberg, A.; Garcia, D.; Suri, G.; Halperin, E.; Gross, J. The Psychology of Collective Emotions. In Current Directions in Psychological Science; SAGE Publications: Thousand Oaks, CA, USA, 2017. [Google Scholar] [CrossRef]
- Peters, K.; Kashima, Y. A multimodal theory of affect diffusion. Psychol. Bull. 2015, 141, 966–992. [Google Scholar] [CrossRef] [PubMed]
- Deli, E.; Peters, J.; Kisvarday, Z. The thermodynamics of cognition: A Mathematical Treatment. Comput. Struct. Biotechnol. J. 2021, 19, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Déli, E.; Kisvárday, Z. The thermodynamic brain and the evolution of intellect: The role of mental energy. Cogn. Neurodyn. 2020, 14, 743–756. [Google Scholar] [CrossRef]
- Chalmers, D. Facing up to the hard problem of consciousness. J. Conscious. Stud. 1995, 2, 200–219. [Google Scholar]
- Singer, W. Recurrent dynamics in the cerebral cortex: Integration of sensory evidence with stored knowledge. Proc. Natl. Acad. Sci. USA 2021, 118, e2101043118. [Google Scholar] [CrossRef]
- Czégel, D.; Giaffar, H.; Csillag, M.; Futó, B.; Szathmáry, E. Novelty and imitation within the brain: A Darwinian neurodynamic approach to combinatorial problems. Sci. Rep. 2021, 11, 12513. [Google Scholar] [CrossRef]
- Itti, L.; Baldi, P. Bayesian surprise attracts human attention. Vis. Res. 2009, 49, 1295–1306. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, H.; Kawamata, O.; Ueda, K. Modeling Emotions Associated with Novelty at Variable Uncertainty Levels: A Bayesian Approach. Front. Comput. Neurosci. 2019, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.; Ashby, W. Every Good Regulator of a System Must Be a Model of That System. In Facets of Systems Science; International Federation for Systems Research; International Series on Systems Science and Engineering 7; Springer: Boston, MA, USA, 1991. [Google Scholar]
- Luck, S.; Gaspelin, N.; Folk, C.; Remington, R.; Theeuwes, J. Progress toward Resolving the Attentional Capture Debate. Vis. Cogn. 2021, 29, 1–21. [Google Scholar] [CrossRef]
- Papazacharias, A.; Taurisano, P.; Fazio, L.; Gelao, B.; Di Giorgio, A.; Lo Bianco, L.; Quarto, T.; Mancini, M.; Porcelli, A.; Romano, R.; et al. Aversive emotional interference impacts behavior and prefrontal-striatal activity during increasing attentional control. Front. Behav. Neurosci. 2015, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Schwarz, N. How one thing leads to another: Spillover effects of behavioral mind-sets. Curr. Dir. Psychol. Sci. 2018, 27, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Flesch, T.; Juechems, K.; Dumbalska, T.; Saxe, A.; Summerfield, C. Orthogonal representations for robust context-dependent task performance in brains and neural networks. Neuron 2022, 110, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Allahverdyan, A.; Galstyan, A.; Abbas, A.; Struzik, Z. Adaptive decision making via entropy minimization. Int. J. Approx. Reason. 2018, 103, 270–287. [Google Scholar] [CrossRef] [Green Version]
- Sedighimornani, N. Is Shame Managed Through Mind-Wandering? Eur. J. Psychol. 2019, 15, 717–732. [Google Scholar] [CrossRef] [Green Version]
- Grossberg, S.; Levine, D.S. Neural dynamics of attentionally modulated Pavlovian conditioning: Blocking, inter-stimulus interval, and secondary reinforcement. Appl. Opt. 1987, 26, 5015–5030. [Google Scholar] [CrossRef]
- Fry, R. Physical Intelligence and Thermodynamic Computing. Entropy 2017, 19, 107. [Google Scholar] [CrossRef]
- Street, S. Upper Limit on the Thermodynamic Information Content of an Action Potential. Front. Comput. Neurosci. 2020, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Hylton, T. Thermodynamic Neural Network. Entropy 2020, 22, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Déli, E.; Peters, J.; Tozzi, A. The Thermodynamic Analysis of Neural Computation. J. Neurosci. Clin. Res. 3 2018, 1, 2. [Google Scholar]
- O’Neill, J.; Schoth, A. The Mental Maxwell Relations: A Thermodynamic Allegory for Higher Brain Functions. Front. Neurosci. 2022, 16, 827888. [Google Scholar] [CrossRef] [PubMed]
- Deli, E. Can the fermionic mind hypothesis (FMH) explain consciousness? The physics of selfhood. Act. Nerv. Super. 2020, 62, 35–47. [Google Scholar] [CrossRef]
- Deli, E. The thermodynamic implications of the fermionic mind hypothesis (FMH). Act. Nerv. Super. 2020, 62, 96–103. [Google Scholar] [CrossRef]
- Hohwy, J. The Predictive Mind; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Schoeller, F.; Perlovsky, L.; Arseniev, D. Physics of mind: Experimental confirmations of theoretical predictions. Phys. Life Rev. 2018, 25, 45–68. [Google Scholar] [CrossRef]
- Parmentier, F.B.R.; Maybery, M.T.; Elsley, J.V. The involuntary capture of attention by novel feature pairings: A study of voice location integration in auditory sensory memory. Atten. Percept. Psychophys. 2010, 72, 279–284. [Google Scholar] [CrossRef]
- Oohashi, T.; Nishina, E.; Honda, M.; Yonekura, Y.; Fuwamoto, Y.; Kawai, N.; Maekawa, T.; Nakamura, S.; Fukuyama, H.; Shibasaki, H. Inaudible high-frequency sounds affect brain activity: Hypersonic effect. J. Neurophysiol. 2000, 83, 3548–3558. [Google Scholar] [CrossRef]
- McCraty, R.; Atkinson, M. Electrophysiology of intuition: Prestimulus responses in group and individual participants using a roulette paradigm. Glob. Adv. Health Med. 2014, 3, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Mohr, C.; Michel, C.; Lantz, G.; Ortigue, S.; Viaud-Delmon, I.; Landis, T. Brain state-dependent functional hemispheric specialization in men but not in women. Cereb. Cortex 2005, 15, 1451–1458. [Google Scholar] [CrossRef]
- Marton, T.; Seifikar, H.; Luongo, F.; Lee, A.T.; Sohal, V. Roles of prefrontal cortex and mediodorsal thalamus in task engagement and behavioral flexibility. J. Neurosci. 2018, 38, 2569–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M.; Raichle, M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Desantis, A.; Waszak, F. Attenuation of auditory N1 results from identity specific action-effect prediction. Eur. J. Neurosci. 2013, 37, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Lange, M.D. Prior expectations induce prestimulus sensory templates. Proc. Natl. Acad. Sci. USA 2017, 114, 10473–10478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uithol, S.; Schurger, A. Reckoning the moment of reckoning in spontaneous voluntary movement. Proc. Natl. Acad. Sci. USA 2016, 4, 817–819. [Google Scholar] [CrossRef] [Green Version]
- Davis, Z.; Muller, L.; Martinez-Trujillo, J.; Sejnowski, T.; Reynolds, J. Spontaneous travelling cortical waves gate perception in behaving primates. Nature 2020, 587, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Chavane, F.; Reynolds, J.; Sejnowski, T. Cortical travelling waves: Mechanisms and computational principles. Nat. Rev. Neurosci. 2018, 19, 255–268. [Google Scholar] [CrossRef]
- Guterstam, A.; Abdulkarim, Z.; Ehrsson, H.H. Illusory ownership of an invisible body reduces autonomic and subjective social anxiety responses. Sci. Rep. 2015, 5, 9831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northoff, G.; Wainio-Theberge, S.; Evers, K. Is temporo-spatial dynamics the “common currency” of brain and mind? In Quest of Spatiotemporal Neuroscience. Phys. Life Rev. 2020, 33, 34–54. [Google Scholar] [CrossRef]
- Wolff, A.; Di Giovanni, D.A.; Gómez-Pilar, J.; Nakao, T.; Huang, Z.; Longtin, A.; Northoff, G. The temporal signature of self: Temporal measures of resting-state EEG predict self-consciousness. Hum. Brain Mapp. 2019, 40, 789–803. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Priestley, J.B.; Castro, A.; Stefanini, F.; Canales, A.S.S.; Balough, E.M.; Lavoie, E.; Mazzucato, L.; Fusi, S.; Losonczy, A. Hippocampal Network Reorganization Underlies the Formation of a Temporal Association Memory. Neuron 2020, 107, 283–291.e6. [Google Scholar] [CrossRef] [PubMed]
- Dörrenbächer, S.; Schütz, C.; Woirgardt, M.; Wu, C.C.; Zimmer, H.D.; Kray, J. Spatio-Temporal Neural Changes after Task-Switching Training in Old Age. Front. Aging Neurosci. 2019, 11, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzulo, G.; Zorzi, M.; Corbetta, M. The secret life of predictive brains: What’s spontaneous activity for? Trends Cogn. Sci. 2021, 25, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.H.; Drissi-Daoudi, L.; Doerig, A. All in Good Time: Long-Lasting Postdictive Effects Reveal Discrete Perception. Trends Cogn. Sci. 2020, 24, 826–837. [Google Scholar]
- Chen, Y.-C.; Spence, C. Assessing the role of the ‘unity assumption’ on multisensory integration: A review. Front. Psychol. 2017, 8, 445. [Google Scholar] [CrossRef] [Green Version]
- Mancini, F.; Longo, M.R.; Kammers, M.P.M.; Haggard, P. Visual distortion of body size modulates pain perception. Psychol. Sci. APS 2011, 22, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Prentner, R. Consciousness and topologically structured spaces. Conscious. Cogn. 2019, 70, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Biderman, N.; Bakkour, A.; Shohamy, D. What Are Memories For? The Hippocampus Bridges Past Experience with Future Decisions. Trends Cogn. Sci. 2020, 24, 542–556. [Google Scholar] [CrossRef]
- Deli, E. The Science of Consciousness; Nadir-Video: USA, 2015; ISBN 10: 9631226263. [Google Scholar]
- Wissner-Gross, D.; Freer, C.E. Causal Entropic Forces. Phys. Rev. Lett. 2013, 110, 168702. [Google Scholar] [CrossRef] [Green Version]
- Andrieux, D.; Gaspard, P.; Ciliberto, S.; Garnier, N.; Joubaud, S.; Petrosyan, A. Entropy production and time asymmetry in nonequilibrium fluctuations. Phys. Rev. Lett. 2007, 98, 150601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspard, P. Brownian motion, dynamical randomness and irreversibility. New J. Phys. 2005, 7, 77. [Google Scholar] [CrossRef]
- Roldán, É.; Parrondo, J.M. Estimating dissipation from single stationary trajectories. Phys. Rev. Lett. 2010, 105, 150607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostic, M. The Elusive Nature of Entropy and Its Physical Meaning. Entropy 2014, 16, 953–967. [Google Scholar] [CrossRef] [Green Version]
- Halgren, A.; Siegel, Z.; Golden, R.; Bazhenov, M. Multielectrode Cortical Stimulation Selectively Induces Unidirectional Wave Propagation in Biophysical/Neural Model. bioRxiv 2020. [Google Scholar] [CrossRef]
- Roberts, J.A.; Gollo, L.L.; Abeysuriya, R.G.; Roberts, G.; Mitchell, P.B.; Woolrich, M.W.; Breakspear, M. Metastable brain waves. Nat. Commun. 2019, 10, 1056. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, W.; Du, Z.; Nadtochiy, A. Regional synapse gain and loss accompany memory formation in larval zebrafish. Proc. Natl. Acad. Sci. USA 2022, 119, e2107661119. [Google Scholar] [CrossRef]
- Sîrbu, A.; Loreto, V.; Servedio, V.; Tria, F. Opinion dynamics: Models, extensions and external effects. In Participatory Sensing, Opinions and Collective Awareness; Springer: Cham, Switzerland, 2017. [Google Scholar]
- León-Medina, F. Endogenous Changes in Public Opinion Dynamics. J. Artif. Soc. Soc. Simul. 2019, 22, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Dankowicz, H. Impact of temporal network structures on the speed of consensus formation in opinion dynamics. Phys. A 2018, 523, 1355–1370. [Google Scholar] [CrossRef] [Green Version]
- Ishii, A.; Kawahata, Y. Opinion Dynamics Theory for Analysis of Consensus Formation and Division of Opinion on the Internet. arXiv 2018, arXiv:1812.11845. [Google Scholar]
- Zha, Q.; Kou, G.; Zhang, H.; Liang, H.; Chen, X.; Li, C.-C.; Dong, Y. Opinion dynamics in finance and business: A literature review and research opportunities. Financial Innov. 2021, 6, 44. [Google Scholar] [CrossRef]
- Vázquez, F.; Saintier, N.; Pinasco, J. Role of voting intention in public opinion polarization. Phys. Rev. E 2020, 101, 012101. [Google Scholar] [PubMed] [Green Version]
- Salehi, S.; Taghiyareh, F. Introspective Agents in Opinion Formation Modeling to Predict Social Market. In Proceedings of the 5th International Conference on Web Research (ICWR), Tehran, Iran, 24–25 April 2019. [Google Scholar]
- Chatterjee, A.; Iannacchione, G. Time and Thermodynamics Extended Discussion on “Time & clocks: A thermodynamic approach”. Results Phys. 2020, 17, 103165. [Google Scholar]
- Zanin, M.; Güntekin, B.; Aktürk, T.; Hanoğlu, L.; Papo, D. Time Irreversibility of Resting-State Activity in the Healthy Brain and Pathology. Front. Physiol. 2020, 10, 16191. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Beaty, R.; Chen, Q.; Sun, J.; Wei, D.; Yang, W.; Qiu, J. Brain Entropy is Associated with Divergent Thinking. Cereb. Cortex 2019, 30, 708–717. [Google Scholar] [CrossRef]
- Saxe, G.; Calderone, D.; Morales, L. Brain entropy and human intelligence: A resting-state fMRI study. PLoS ONE 2018, 13, e0191582. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Chang, D.; Zhang, J.; Ge, Q.; Zang, Y.; Wang, Z. Associations of brain entropy (BEN) to cerebral blood flow and fractional amplitude of low-frequency fluctuations in the resting brain. Brain Imaging Behav. 2018, 13, 1486–1495. [Google Scholar] [CrossRef]
- McIntosh, A.R.; Kovacevic, N.; Itier, R. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 2008, 4, e1000106. [Google Scholar] [CrossRef] [Green Version]
- Kolvoort, I.R.; Wainio-Theberge, S.; Wolff, A.; Northoff, G. Temporal integration as “common currency” of brain and self-scale-free activity in resting-state EEG correlates with temporal delay effects on self-relatedness. Hum. Brain Mapp. 2020, 41, 4355–4374. [Google Scholar] [CrossRef]
- Spinhoven, P.; van Hemert, A.; Penninx, B. Repetitive negative thinking as a predictor of depression and anxiety: A longitudinal cohort study. J. Affect. Disord. 2018, 241, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? NeuroImage 2011, 57, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.; Paré, D. (Eds.) The brain as a closed system modulated by the senses. In The Churchlands and Their Critics; Blackwell Publishers: Cambridge, MA, USA, 1996. [Google Scholar]
- Northoff, G. Is Our Brain an Open or Closed System? Prediction Model of Brain and World–Brain Relation. In The Spontaneous Brain; MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Deli, E.; Peters, J.; Tozzi, A. Relationships between short and fast brain timescales. Cogn. Neurodynamics 2017, 11, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Bale, G.; Mitra, S.; Meek, J.; Robertson, N.; Tachtsidis, I. A new broadband near-infrared spectroscopy system for in-vivo measurements of cerebral cytochrome-c-oxidase changes in neonatal brain injury. Biomed. Opt. Express 2014, 5, 3450–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowen, A.; Sauter, D.; Tracy, J.; Keltner, D. Mapping the Passions: Toward a High-Dimensional Taxonomy of Emotional Experience and Expression. Psychol. Sci. Public Interest 2019, 20, 69–90. [Google Scholar] [CrossRef]
- Keltner, D.; Sauter, D.; Tracy, J.; Cowen, A. Emotional Expression: Advances in Basic Emotion Theory. J. Nonverbal Behav. 2019, 43, 133–160. [Google Scholar] [CrossRef]
- Gothard, M. Marmosets confirm that context is king. Neuron 2022, 110, 1273–1274. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Wu, J.; Mashour, G.A.; Hudetz, A.G. Temporal circuit of macroscale dynamic brain activity supports human consciousness. Sci. Adv. 2020, 6, eaaz0087. [Google Scholar] [CrossRef] [Green Version]
- Schoeller, F.; Perlovsky, L. Aesthetic Chills: Knowledge-Acquisition, Meaning-Making, and Aesthetic Emotions. Front. Psychol. 2016, 7, 1093. [Google Scholar] [CrossRef] [Green Version]
- Krause, S.; Bornholdt, S. Opinion formation model for markets with a social temperature and fear. Phys. Rev. E 2012, 86 Pt 2, 056106. [Google Scholar] [CrossRef] [Green Version]
- Kao, F.-C.; Wang, S.; Chang, Y. Brainwaves Analysis of Positive and Negative Emotions. ISAA 2015, 12, 1263–1266. [Google Scholar]
- Gao, Z.; Cui, X.; Wan, W.; Zheng, W.; Gu, Z. Long-range correlation analysis of high frequency prefrontal electroencephalogram oscillations for dynamic emotion recognition. Biomed. Signal Process. Control 2022, 72, 103291. [Google Scholar] [CrossRef]
- Hesp, C.; Smith, R.; Parr, T.; Allen, M.; Friston, K.J.; Ramstead, M.J.D. Deeply Felt Affect: The Emergence of Valence in Deep Active Inference. Neural Comput. 2021, 33, 398–446. [Google Scholar] [CrossRef]
- Joffily, M.; Coricelli, G. Emotional valence and the free-energy principle. PLoS Comput. Biol. 2013, 9, e1003094. [Google Scholar] [CrossRef] [Green Version]
- Buzsaki, N. Logothetis and W. Singer. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron 2013, 80, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Esghaei, M.; Treue, S.; Vidyasagar, T. Dynamic coupling of oscillatory neural activity and its roles in visual attention. Trends Neurosci. 2022, 45, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Bechler, C.J.; Tormala, Z.; Rucker, D. Perceiving attitude change: How qualitative shifts augment change perception. J. Exp. Soc. Psychol. 2019, 82, 160–175. [Google Scholar] [CrossRef]
- Stringer, C.; Pachitariu, M.; Steinmetz, N.; Reddy, C.B.; Carandini, M.; Harris, K.D. Spontaneous behaviors drive multidimensional, brainwide activity. Science 2019, 364, eaav7893. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, Y.L.H.; Song, L. Social Media and Attitude Change: Information Booming Promote or Resist Persuasion? Front. Psychol. 2021, 12, 2433. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazzaz, N.; Sabir, M.; Ali, S.; Ahmad, S.; Grammer, K. Electroencephalogram Profiles for Emotion Identification over the Brain Regions Using Spectral, Entropy and Temporal Biomarkers. Sensors 2019, 20, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bányai, M.E.A. Stimulus complexity shapes response correlations in primary visual cortex. Proc. Natl. Acad. Sci. USA 2019, 116, 2723–2732. [Google Scholar] [CrossRef] [Green Version]
- Moca, V.V.; Nagy-Dabacan, A.; Harzan, H.B.; Muresan, R.C. Superlets: Time-frequency super-resolution using wavelet sets. bioRxiv 2019, 583732. [Google Scholar] [CrossRef] [Green Version]
- Lutas, A.; Kucukdereli, H.; Alturkistani, O.; Carty, C.; Sugden, A.U.; Fernando, K.; Diaz, V.; Flores-Maldonado, V.; Andermann, M.L. State-specific gating of salient cues by midbrain dopaminergic input to basal amygdala. Nat. Neurosci. 2019, 22, 1820–1833. [Google Scholar] [CrossRef]
- Li, H.; Namburi, P.; Olson, J.M.; Borio, M.; Lemieux, M.E.; Beyeler, A.; Calhoon, G.G.; Hitora-Imamura, N.; Coley, A.A.; Libster, A.; et al. Neurotensin orchestrates valence assignment in the amygdala. Nature 2022, 608, 586–592. [Google Scholar] [CrossRef]
- Tozzi, A.; Peters, J.F. From abstract topology to real thermodynamic brain activity. Cogn. Neurodyn. 2017, 11, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.; Peters, J.F. A topological approach unveils system invariances and broken symmetries in the brain. J. Neurosci. Res. 2016, 94, 351–365. [Google Scholar] [CrossRef]
- Don, A.P.H.; Peters, J.; Ramanna, S.; Tozzi, A. Topological view of flows inside the BOLD spontaneous activity of the human brain. Front. Comput. Neurosci. 2020, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Lubashevsky, I. Psychophysical laws as reflection of mental space properties. Phys. Life Rev. 2019, 31, 276–303. [Google Scholar] [CrossRef] [Green Version]
- Tsao, A.; Sugar, J.; Lu, L.; Wang, C.; Knierim, J.J.; Moser, M.B.; Moser, E.I. Integrating time from experience in the lateral entorhinal cortex. Nature 2018, 25, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Babaev, O.; Chatain, C.P.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Vogel, E.; Krabbe, S.; Gründemann, J.; Cusulin, J.; Lüthi, A. Projection-Specific Dynamic Regulation of Inhibition in Amygdala Micro-Circuits. Neuron 2016, 91, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, W.; Kochiyama, T.; Uono, S.; Sawada, R.; Yoshikawa, S. Amygdala activity related to perceived social support. Sci. Rep. 2020, 10, 2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fingelkurts, A.A.; Fingelkurts, A.A. Present moment, past, and future: Mental kaleidoscope. Front. Psychol. 2014, 5, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Steenbergen, H.; Band, G.; Hommel, B. Threat but not Arousal Narrows Attention: Evidence from Pupil Dilation and Saccade Control. Front. Psychol. 2011, 2, 281. [Google Scholar] [CrossRef] [Green Version]
- Parr, T.; Rees, G.; Friston, K. Computational Neuropsychology and Bayesian Inference. Front. Hum. Neurosci. 2018, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Crawford, L.E.; Knouse, L.; Kent, M.; Vavra, D.; Harding, O.; Leserve, D.; Fox, N.; Hu, X.; Li, P.; Glory, C.; et al. Enriched environment exposure accelerates rodent driving skills. Behav. Brain Res. 2000, 378, 112309. [Google Scholar] [CrossRef]
- Neal, S.; Kent, M.; Bardi, M.; Lambert, K.G. Enriched Environment Exposure Enhances Social Interactions and Oxytocin Responsiveness in Male Long-Evans Rats. Front. Behav. Neurosci. 2018, 12, 198. [Google Scholar] [CrossRef]
- Planchez, B.; Lagunas, N.; Le Guisquet, A.M.; Legrand, M.; Surget, A.; Hen, R.; Belzung, C. Increasing Adult Hippocampal Neurogenesis Promotes Resilience in a Mouse Model of Depression. Cells 2021, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, R.; Wu, J.; Yang, Q.; Zheng, S.; Wu, K. Psychological resilience, self-acceptance, perceived social support and their associations with mental health of incarcerated offenders in China. Asian J. Psychiatry 2020, 52, 102166. [Google Scholar] [CrossRef]
- Jans-Beken, L.; Jacobs, N.; Janssens, M.; Peeters, S.; Reijnders, J.; Lechner, L.; Lataster, J. Gratitude and health: An updated review. J. Posit. Psychol. 2019, 15, 743–782. [Google Scholar] [CrossRef]
- Ng, R.; Allore, H.G.; Levy, B.R. Self-Acceptance and Interdependence Promote Longevity: Evidence From a 20-year Prospective Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 5980. [Google Scholar] [CrossRef]
- Roy, D.S.; Park, Y.G.; Kim, M.E.; Zhang, Y.; Ogawa, S.K.; DiNapoli, N.; Gu, X.; Cho, J.H.; Choi, H.; Kamentsky, L.; et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat. Commun. 2022, 13, 1799. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Ye, L.; Yang, Q.; Cui, Q.; He, Z.; Li, L.; Yang, X.; Zou, Q.; Yang, P.; et al. Spatial complexity of brain signal is altered in patients with generalized anxiety disorder. J. Affect. Disord. 2018, 246, 387–393. [Google Scholar] [CrossRef]
- Inzlicht, M.; Bartholow, B.D.; Hirsh, J.B. Emotional foundations of cognitive control. Trends Cogn. Sci. 2015, 19, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Alzheimer’s Disease Neuroimaging Initiative. Brain Entropy Mapping in Healthy Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 596122. [Google Scholar] [CrossRef]
- Gustavson, D.E.; du Pont, A.; Whisman, M.A.; Miyake, A. Evidence for Transdiagnostic Repetitive Negative Thinking and Its Association with Rumination, Worry, and Depression and Anxiety Symptoms: A Commonality Analysis. Collabra Psychol. 2018, 4, 13. [Google Scholar] [CrossRef]
- Mohammadkhani, S.; Attar, F.; Akbari, M. The linkage between negative affectivity with emotional distress in college student: The mediator and moderator role of difficulty in emotion regulation, repetitive negative thinking, and emotional invalidation. Curr Psychol. 2021, 1–13. [Google Scholar] [CrossRef]
- Saarimäki, H.; Ejtehadian, L.F.; Glerean, E.; Vuilleumier, P.; Sams, M.; Nummenmaa, L. Distributed affective space represents multiple emotion categories across the brain. bioRxiv 2017, 123521. [Google Scholar] [CrossRef] [PubMed]
- Trevisiol, A.; Saab, A.S.; Winkler, U.; Marx, G.; Imamura, H.; Möbius, W.; Kusch, K.; Nave, K.A.; Hirrlinger, J. Monitoring ATP dynamics in electrically active white matter tracts. eLife 2017, 6, e24241. [Google Scholar] [CrossRef] [PubMed]
- Laws, B. The return of the suppressed: Exploring how emotional suppression reappears as violence and pain among male and female prisoners. Punishm. Soc. 2019, 21, 560–577. [Google Scholar] [CrossRef]
- London, E.D. Impulsivity, stimulant abuse, and dopamine receptor signaling. Adv. Pharmacol. 2016, 76, 67–84. [Google Scholar]
- Remmers, C.; Zander, T. Why you don’t see the forest for the trees when you are anxious: Anxiety impairs intuitive decision making. Clin. Psychol. Sci. 2018, 6, 48–62. [Google Scholar] [CrossRef]
- Hollis, F.; van der Kooij, M.A.; Zanoletti, O. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. USA 2015, 112, 15486–15491. [Google Scholar] [CrossRef] [Green Version]
- Gehring, W.J.; Coles, M.G.H.; Meyer, D.E.; Donchin, E. The error-related negativity: An event-related brain potential accompanying errors. Psychophysiology 2018, 27, 534. [Google Scholar]
- Ruan, Y.; Reis, H.; Zareba, W.; Lane, R.D. Does suppressing negative emotion impair subsequent emotions? Two experience sampling studies. Motiv. Emot. 2020, 44, 427–435. [Google Scholar] [CrossRef]
- Lupien, S.; Maheu, F.; Tu, M.; Fiocco, A. The effects of stress and stress hormones on human cognition. Implications for the field of brain and cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef] [Green Version]
- MacInnes, D.L. Self-Esteem and Self-Acceptance: An Examination into Their Relationship and Their Effect on Psychological Health. J. Psychiatr. Ment. Health Nurs. 2006, 13, 483–489. [Google Scholar] [CrossRef]
- Yanagisawa, H. Free-Energy Model of Emotion Potential: Modeling Arousal Potential as Information Content Induced by Complexity and Novelty. Front. Comput. Neurosci. 2021, 15, 698252. [Google Scholar] [CrossRef]
- Jao, C.-W.; Yeh, J.-H.; Wu, Y.-T. Alteration of the Intra- and Inter-Lobe Connectivity of the Brain Structural Network in Normal Aging. Entropy 2020, 22, 826. [Google Scholar] [CrossRef] [PubMed]




| Physical Systems | Brain Activations | |
|---|---|---|
| Microstates orientation | Oriented in space | Information entropy oriented in time |
| System evolution | Brownian motion | Wave-like activations founded on memories |
| Entropic force | Irreversible macroscopic behavior | Irreversible activations |
| The consequences of irreversibility | The arrow of time | Future orientation, novelty, curiosity, and creativity |
| High entropy state | Equilibrium | Equilibrium |
| Consequences of high entropy | Loss of work potential | Intellect, confidence, and a can-do attitude |
| Energy input lowers the entropy | The system moves away from equilibrium, but irreversibility remains! | Reversible and repetitive activations |
| Consequences of low entropy | Increasing work potential | Uncertainty, lack of control, and psychological problems |
| Exothermic Reaction (Mental Energy Loss) | Endothermic Reaction (Mental Energy Gain) | |
|---|---|---|
| High entropy environment (stress) | Spontaneous behavior | Spontaneous on low social temperature, which permits overcoming the negativity |
| Low entropy, supportive environment | Spontaneous on high social temperature because the aggravation overcomes the support | Spontaneous behavior |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Déli, É.; Peters, J.F.; Kisvárday, Z. How the Brain Becomes the Mind: Can Thermodynamics Explain the Emergence and Nature of Emotions? Entropy 2022, 24, 1498. https://doi.org/10.3390/e24101498
Déli É, Peters JF, Kisvárday Z. How the Brain Becomes the Mind: Can Thermodynamics Explain the Emergence and Nature of Emotions? Entropy. 2022; 24(10):1498. https://doi.org/10.3390/e24101498
Chicago/Turabian StyleDéli, Éva, James F. Peters, and Zoltán Kisvárday. 2022. "How the Brain Becomes the Mind: Can Thermodynamics Explain the Emergence and Nature of Emotions?" Entropy 24, no. 10: 1498. https://doi.org/10.3390/e24101498
APA StyleDéli, É., Peters, J. F., & Kisvárday, Z. (2022). How the Brain Becomes the Mind: Can Thermodynamics Explain the Emergence and Nature of Emotions? Entropy, 24(10), 1498. https://doi.org/10.3390/e24101498