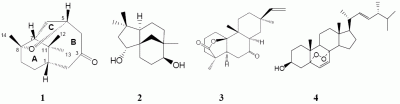
Norcyperone, a Novel Skeleton Norsesquiterpene from Cyperus rotundus L.
Abstract
:Introduction
Results and Discussion




| Position | 1H-NMR | 13C-NMR | HMBC (H→C) |
|---|---|---|---|
| 1 | 1.88 (1H, m) | 38.6 | C-3, 7, 9, 12, 13 |
| 2a | 2.86 (1H, dd, 12.6, 1.8) | 47.9 | C-1, 3, 10 |
| 2b | 2.34 (1H, ddd, 12.6, 7.8, 1.2) | C-1, 3, 4, 10, 11 | |
| 3 | - | 211.3 | |
| 4a | 2.88 (1H, ddd, 13.2,7.8, 1.8) | 52.6 | C-2, 3, 5, 6 |
| 4b | 2.62 (1H, dd, 13.2, 3.0) | C-3, 5, 6 | |
| 5 | 4.39 (1H, ddt, 10.2, 7.2, 1.8) | 72.2 | C-3, 7, 8 |
| 6a | 2.64 (1H, dd, 14.4, 10.2) | 35.4 | C-4, 5, 7, 11 |
| 6b | 2.18 (1H, br d, 14.4) | C-4, 7, 8, 11 | |
| 7 | 1.77 (1H, d, 10.2) | 50.6 | C-1, 5, 12, 14 |
| 8 | - | 83.8 | |
| 9a | 1.85 (1H, m) | 30.3 | C-1, 7, 8, 10 |
| 9b | 1.79 (1H, m) | C-8, 10 | |
| 10a | 1.94 (1H, m) | 19.6 | C-1, 2, 8, 9 |
| 10b | 1.90 (1H, m) | C-1, 2, 8, 9 | |
| 11 | - | 35.1 | |
| 12 | 1.14 (3H, s) | 34.3 | C-1, 7, 11, 13 |
| 13 | 1.11 (3H, s) | 28.4 | C-1, 7, 11, 12 |
| 14 | 1.17 (3H, s) | 30.1 | C-7, 8, 9 |
Conclusions
Experimental
General
Plant material
Extraction and isolation
Crystallographic Data
Acknowledgements
References
- Jiangsu New Medical College. Dictionary of Chinese Materia Medica; Shanghai People’s Publishing House: Shanghai, P.R.China, 1971; pp. 3441–3443. [Google Scholar]
- Raut, N. A.; Gaikwad, N. J. Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 2006, 77, 585–588. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, R. Cyperus rotundus extract inhibits acetylcholinesterase activity from animal and plants as well as inhibits germination and seedling growth in wheat and tomato. Life Sci. 2007, 80, 2389–2392. [Google Scholar] [CrossRef]
- Uddin, S.J.; Mondal, K.; Shilpi, J. A.; Rahman, M. T. Antidiarrhoeal activity of Cyperus rotundus. Fitoterapia 2006, 77, 134–136. [Google Scholar] [CrossRef]
- Seo, W. G.; Pae, H. O.; Oh, G. S.; Chai, K. Y.; Kwon, T.O.; Yun, Y. G.; Kim, N. Y.; Chung, H. T. Inhibitory effects of methanol extract of Cyperus rotundus rhizomes on nitric oxide and superoxide productions by murine macrophage cell line, RAW 264.7 cells. J. Ethnopharmacol. 2001, 76, 59–64. [Google Scholar] [CrossRef]
- Huang, X. F.; Peng, G. P. Research progress on chemical components and the pharmacology of the rhizomes of Cyperus rotundus L. J. Chin. Med. Mate. 2003, 26, 65–68. [Google Scholar]
- Jeong, S. J.; Miyamoto, T.; Inagaki, M.; Kim, Y.C.; Higuchi, R. Rotundines A-C, three novel sesquiterpene alkaloids from Cyperus rotundus. J. Nat. Prod. 2000, 63, 673–675. [Google Scholar] [CrossRef]
- Weehen, H.; Nkunya, M. H. H.; Bray, D. H.; Mwasumbi, L. B.; Kinabo, L. S.; Kilimali, A. E.; Wijnberg, B. P. A. Antimalarial activity of Tanzanian plants. Part 2. Antimalarial compounds containing an α,β-unsaturated carbonyl moiety from Tanzanian medicinal plants. Planta Med. 1990, 56, 371–373. [Google Scholar] [CrossRef]
- Sayed, H. M.; Mohamed, M.H.; Farag, S.F.; Mohamed, G.A.; Proksch, P. A new steroid glycoside and furochromones from Cyperus roundus L. Nat. Prod. Res. 2007, 21, 343–350. [Google Scholar] [CrossRef]
- Tsui, W. Y.; Brown, G. D. Sesquiterpenes from Baeckea frutescens. J. Nat. Prod. 1996, 59, 1084–1086. [Google Scholar] [CrossRef]
- Loukaci, A.; Kayser, O.; Bindseil, K. U.; Siems, K.; Frevert, J.; Abreu, P. M. New trichothecenes isolated from Holarrhena floribunda. J. Nat. Prod. 2000, 63, 52–56. [Google Scholar] [CrossRef]
- Ishizuka, T.; Yaoita, Y.; Kikuehi, M. Sterols from the fruit body of Grifola frondosa. Chem. Pharm. Bull. 1997, 45, l756–1760. [Google Scholar] [CrossRef]
- Li, G. Y.; Li, B. W.; Liu, G. Y.; Zhang, G. L. Sterols from Aspergillus ocharceus 43. Chin. J. Appl. Environ. Biol. 2005, 11, 67–70. [Google Scholar]
- Friese, J. C.; Schafer, H. J. Short synthesis of a taxane-AB-fragment with a spiro-cyclopropyl group. Synlett. 2002, 5, 814–816. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, Y.; Zhang, H.-W.; Yu, C.-Y.; Lu, Y.; Chang, Y.; Zou, Z.-M. Norcyperone, a Novel Skeleton Norsesquiterpene from Cyperus rotundus L. Molecules 2008, 13, 2474-2481. https://doi.org/10.3390/molecules13102474
Xu Y, Zhang H-W, Yu C-Y, Lu Y, Chang Y, Zou Z-M. Norcyperone, a Novel Skeleton Norsesquiterpene from Cyperus rotundus L. Molecules. 2008; 13(10):2474-2481. https://doi.org/10.3390/molecules13102474
Chicago/Turabian StyleXu, Yan, Hong-Wu Zhang, Chang-Yuan Yu, Yang Lu, Ying Chang, and Zhong-Mei Zou. 2008. "Norcyperone, a Novel Skeleton Norsesquiterpene from Cyperus rotundus L." Molecules 13, no. 10: 2474-2481. https://doi.org/10.3390/molecules13102474
APA StyleXu, Y., Zhang, H. -W., Yu, C. -Y., Lu, Y., Chang, Y., & Zou, Z. -M. (2008). Norcyperone, a Novel Skeleton Norsesquiterpene from Cyperus rotundus L. Molecules, 13(10), 2474-2481. https://doi.org/10.3390/molecules13102474