Solid-Phase Synthesis of Optically Active Substituted 2 Aminofuranones Using an Activated Carbonate Linker
Abstract
:Introduction


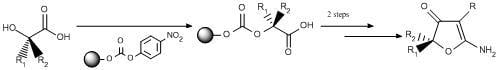
Results and Discussion
| Product | R1 | R2 | R3 | %yield |
|---|---|---|---|---|
| 1a | CO2Et | H | H | 40 |
| 1b | CN | H | H | 52 |
| 1c | CO2Et | Me | H | 37 |
| 1d | CN | Me | H | 52 |
| 1e | CO2Et | Ph | H | 58 |
| 1f | CN | Ph | H | 43 |
| 1g | CO2Et | Me | Me | 53 |
| 1h | CN | Me | Me | 54 |
Conclusions
Experimental
General
General procedure for the synthesis of 3,5-disubstituted-2-aminofuranones
Acknowledgements
References and Notes
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Krchnak, V.; Holladay, M.W. Solid phase heterocyclic chemistry. Chem. Rev. 2002, 102, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, A.; Ostresh, J.M.; Houghten, R.A. The Current Status of Heterocyclic Combinatorial Libraries. Chem. Rev. 1997, 97, 449–472. [Google Scholar] [CrossRef] [PubMed]
- Franzen, R.G. Recent advances in the preparation of heterocycles on solid support: a review of the literature. J. Comb. Chem. 2000, 195–214. [Google Scholar] [CrossRef]
- Brown, A.R.; Hermkerns, P.H.H.; Ottenheijm, H.C.J.; Rees, D.C. Solid phase synthesis. Synlett 1998, 817–827. [Google Scholar] [CrossRef]
- Ganesan, A. Methods in Enzymology, Vol. 369: Combinatorial Chemistry, Part B; Morales, G.A., Bunin, B.A., Eds.; Elsevier: San Diego, CA, USA, 2003; pp. 415–434. [Google Scholar]
- Pernerstorfer, J. Combinatorial Chemistry: From Theory to Application; Bannwarth, W., Hinzen, B., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 111–142. [Google Scholar]
- Jung, N.; Wiehn, M.; Braese, S. Multifunctional Linkers for Combinatorial Solid Phase Synthesis. Top. Curr. Chem. 2007, 278, 1–88. [Google Scholar]
- Scott, P.J.H.; Steel, P.G. Diversity Linker Units for Solid Phase Organic Synthesis. Eur. J. Org. Chem. 2006, 2251–2268. [Google Scholar] [CrossRef]
- Tersteegen, A.; Heimbach, D.; Thede, K.; Welker, R.; Fast, B.; Paessens, A.; Dittmer, F.; Schohe-Loop, R.; Harrenga, A.; Hillisch, A.; Henninger, K.; Huebsch, W.; Bauser, M.; Greschat, S.; Schneider, D.; Marquardt, T.; Goeller, A.; Urban, A.; Wildum, S.; Paulsen, D. Preparation of aminofuranones as HIV-1 protease inhibitors. WO 2008071359 2008. [Google Scholar]
- Buehler, H.; Bayer, A.; Effenberger, F. A convenient synthesis of optically active 5,5-disubstituted 4-amino- and 4-hydroxy-2(5H)-furanones from (S)-ketone cyanohydrins. Chem. Eur. J. 2000, 2564–2571. [Google Scholar] [CrossRef]
- Menta, E.; Pescalli, N.; Conti, M.; Zimmerman, G. Ureido and thioureido derivatives of 4-amino-2(5H)-furanones and 4-amino-2(5H)-thiophenones as antitumor agents. US Patent 6333346 2001. [Google Scholar]
- Hiyama, T.; Oishi, H.; Suetsugu, Y.; Nishide, K.; Saimoto, H. Synthesis of 4-amino-2(5H)-furanones through intra- and intermolecular nitrile addition of ester enolates. Construction of carbon framework of an antitumor antibiotic basidalin. Bull. Chem. Soc. Jpn. 1987, 60, 2139–2150. [Google Scholar] [CrossRef]
- Lattmann, E.; Dunn, S.; Niamsanit, S.; Sattayasai, N. Synthesis and antibacterial activities of 5-hydroxy-4-amino-2(5H)-furanones. Bioorg. Med. Chem. Lett. 2005, 15, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Mavrov, M.; Simirskaya, N. Synthesis of substituted 3-aminospiro-4-but-2-enolides. Chem. Heter. Comp. 2000, 35, 1150–1155. [Google Scholar] [CrossRef]
- Pevet, I.; Meyer, C.; Cossy, J. [2,3]-Wittig sigmatropic rearrangement of γ-allyloxy-β-enaminoesters. Synlett 2003, 5, 663–666. [Google Scholar] [CrossRef]
- Hiyama, T.; Oishi, H.; Saimoto, H. New synthetic methods for 4-amino-2(5H)-furanones. Tetrahedron Lett. 1985, 26, 2459–2462. [Google Scholar] [CrossRef]
- Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Procopio, A.; Rescifina, A.; Romeo, G.; Romeo, R. Intramolecular cycloadditions of alpha-allyloxycarbonylnitrones: Stereoselective synthesis of 3-amino-2(5H)furanones. J. Org. Chem. 2002, 67, 4380–4383. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G.; Ward, C.E.; Lo, W.C.; Nagy, J.O.; Boeger, P. Bleaching herbicide flurtamone interferes with phytoene desaturase. Plant Physiol. 1990, 94, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.E.; Lo, W.C.; Pomidor, P.B.; Tisdell, F.E.; Ho, A.W.W.; Chiu, C.L.; Tuck, D.M.; Bernando, C.R.; Fong, P.J.; Omid, A.; Buteau, F.A. 5-Aminofuran-3(2H)-ones. A new development in bleaching herbicides. In Synthesis and Chemistry of Agrochemicals; ACS Symposium Series, No. 35; Baker, D.R., Fenyes, J.G., Moberg, W.K., Barrington, C., Eds.; American Chemical Society: Washington, DC, USA, 1987; pp. 65–73. [Google Scholar]
- Athanasellis, G.; Detsi, A.; Prousis, K.C.; Igglessi-Markopoulou, O. Novel access to 2-aminofuranones via cyclization of functionalized γ-hydroxy-α,β-butenoates derived from N-hydroxybenzotriazole esters of α-hydroxy acids. Synthesis 2003, 2015–2022. [Google Scholar] [CrossRef]
- Athanasellis, G.; Gavrielatos, E.; Igglessi-Markopoulou, O. One-pot synthesis of optically active tetramic acids from amino acids mediated by 1-hydroxybenzotriazole. Synlett 2001, 10, 1653–1655. [Google Scholar] [CrossRef]
- Kikionis, S.; McKee, V.; Markopoulos, J.; Igglessi-Markopoulou, O. Regioselective ring opening of thiomalic acid anhydrides by carbon nucleophiles. Synthesis and X-ray structure elucidation of novel thiophenone derivatives. Tetrahedron 2009, 65, 3711–3716. [Google Scholar] [CrossRef]
- Mitsos, C.A.; Zografos, A.L.; Igglessi-Markopoulou, O. Regioselective Ring Opening of Malic Acid Anhydrides by Carbon Nucleophiles. Application in the Synthesis of Chiral Tetronic Acids. J. Org. Chem. 2000, 65, 5852–5853. [Google Scholar] [CrossRef] [PubMed]
- Prousis, K.; Detsi, A.; Igglessi-Markopoulou, O. A traceless solid-phase approach to functionalized tetramic acids and 2-amino-4-pyrrolinones. Synlett 2005, 18, 2763–2766. [Google Scholar]
- Dressman, B.A.; Spangle, L.A.; Kaldor, S.W. Solid phase synthesis of hydantoins using a carbamate linker and a novel cyclization / cleavage step. Tetrahedron Lett. 1996, 37, 937–940. [Google Scholar] [CrossRef]
- Anschuetz, R. Imidotetronic acid. Ber. Dtsch. Chem. Ges. 1912, 2374–2378. [Google Scholar]
Sample Availability: Contact the authors. |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matiadis, D.; Prousis, K.C.; Igglessi-Markopoulou, O. Solid-Phase Synthesis of Optically Active Substituted 2 Aminofuranones Using an Activated Carbonate Linker. Molecules 2009, 14, 3914-3921. https://doi.org/10.3390/molecules14103914
Matiadis D, Prousis KC, Igglessi-Markopoulou O. Solid-Phase Synthesis of Optically Active Substituted 2 Aminofuranones Using an Activated Carbonate Linker. Molecules. 2009; 14(10):3914-3921. https://doi.org/10.3390/molecules14103914
Chicago/Turabian StyleMatiadis, Dimitris, Kyriakos C. Prousis, and Olga Igglessi-Markopoulou. 2009. "Solid-Phase Synthesis of Optically Active Substituted 2 Aminofuranones Using an Activated Carbonate Linker" Molecules 14, no. 10: 3914-3921. https://doi.org/10.3390/molecules14103914
APA StyleMatiadis, D., Prousis, K. C., & Igglessi-Markopoulou, O. (2009). Solid-Phase Synthesis of Optically Active Substituted 2 Aminofuranones Using an Activated Carbonate Linker. Molecules, 14(10), 3914-3921. https://doi.org/10.3390/molecules14103914