An Unsymmetrical Tetrathiafulvalene with a Fused 1,2,5-Thiadiazole Ring and Methylthio Groups
Abstract
:Introduction

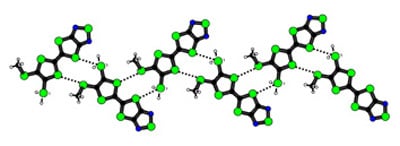
Results and Discussion

| Bond lengths (Å) | Bond angles (°) | ||
|---|---|---|---|
| S1–C1 | 1.756(3) | C2–S1–C1 | 93.97(15) |
| S2–C1 | 1.766(3) | C3–S2–C1 | 93.83(16) |
| S3–N2 | 1.643(3) | N2–S3–N1 | 99.39(15) |
| S3–N1 | 1.649(3) | C5–S4–C4 | 95.28(15) |
| S4–C4 | 1.757(3) | C6–S5–C4 | 95.64(14) |
| S5–C4 | 1.752(3) | C2–N1–S3 | 105.9(3) |
| N1–C2 | 1.318(4) | C3–N2–S3 | 106.2(2) |
| N2–C3 | 1.314(4) | N1–C2–C3 | 114.2(3) |
| C1–C4 | 1.353(4) | N2–C3–C2 | 114.3(3) |
| C2–C3 | 1.428(5) | ||





Conclusions
Experimental
General
X-Ray crystallography
| Chemical formula | C8H6N2S7 |
| Formula weight | 354.64 |
| Temperature | 295(1) K |
| Wavelength | 0.71070 Å |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a | 7.9119(8) Å |
| b | 12.6713(14) Å |
| c | 13.3589(15) Å |
| β | 93.868(1)° |
| V | 1336.2(3) Å3 |
| Z | 4 |
| Calculated density | 1.763 Mgm-3 |
| Absorption coefficient | 1.155 mm-1 |
| F(000) | 720 |
| Crystal size | 0.35 × 0.05 × 0.05 mm |
| θ Range for data collection | 3.04–27.48° |
| Index ranges | –10 ≤ h ≤ 9 |
| –16 ≤ k ≤ 16 | |
| –14 ≤ l ≤ 17 | |
| Completeness to θ | 95.9 % |
| Reflections collected | 12,282 |
| Independent reflections | 2,945 [ Rint = 0.0356] |
| Absorption correction | None |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restrains/parameters | 2,945/0/156 |
| Goodness-of-fit on F2 | 1.186 |
| Final R indices [I > 2σ(I)] | R1 = 0.0547, wR2 = 0.1250 |
| R indices (all data) | R1 = 0.0687, wR2 = 0.1330 |
| Largest diff. peak and hole | 0.483 and –0.362 eÅ-3 |
| CCDC Deposition number | CCDC 675501 |
Theoretical calculations
Acknowledgments
- Sample Availability: Samples of the title compound are available from the authors.
References
- Tomura, M.; Tanaka, S.; Yamashita, Y. Preparation and Properties of Bis[1,2,5]thiadiazolo-tetrathiafulvalene. Heterocycles 1993, 35, 69–72. [Google Scholar] [CrossRef]
- Underhill, A.E.; Hawkins, I.; Edge, S.; Wilkes, S.B. Bis(thiadiazole)tetrathiafulvalene (BTDA-TTF). Synth. Met. 1993, 56, 1914–1919. [Google Scholar]
- Naito, T.; Kobayashi, A.; Kobayashi, H.; Underhill, A. E. New Synthetic Metals Based on a Thiadiazole Network. Chem. Commun. 1996, 521–522. [Google Scholar]
- Yamada, J.; Satoki, S.; Mishima, S.; Akashi, N.; Takahashi, K.; Masuda, N.; Nishimoto, Y.; Takasaki, S.; Anzai, H. Synthesis of Unsymmetrical Tetrathiafulvalene Derivatives via Me3Al-Promoted Reactions of Organotin Compounds with Esters. J. Org. Chem. 1996, 61, 3987–3995. [Google Scholar]
- Tomura, M.; Yamashita, Y. Synthesis, Structure, and Physical Properties of Novel Component Molecules with Fused Heterocycles for Organic Conductor. Synth. Met. 1997, 86, 1871–1872. [Google Scholar] [CrossRef]
- Tomura, M.; Yamashita, Y. Unsymmetrical Tetrathiafulvalene with a Fused 1,2,5-Thiadiazole Ring and an Ethylenedioxy Group. Acta. Cryst. E 2003, 59, o145–o147. [Google Scholar] [CrossRef]
- Tomura, M.; Yamashita, Y. 4,5-Diiodo[1,2,5]thiadiazolotetrathiafulvalene. Acta. Cryst. E 2004, 60, o63–o65. [Google Scholar] [CrossRef]
- Misaki, Y.; Miura, T.; Fujiwara, H.; Kawakami, K.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. BDT-TTP Donors Fused with Aromatic Rings and Their Cation Radical Salts. Synth. Met. 1997, 86, 1821–1822. [Google Scholar] [CrossRef]
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, K.D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.-H. Organic Superconductors (Including Fullerenes); Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Ishiguro, T.; Yamaji, K.; Saito, G. Organic Superconductors, 2nd ed; Springer-Verlag: Berlin, Germany, 1998. [Google Scholar]
- Yamashita, Y.; Tomura, M. Highly Polarized Electron-Donors, Acceptors and Donor-Acceptor Compounds. J. Mater. Chem. 1998, 8, 1933–1944. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths Determined by X-ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar]
- Mellini, M.; Merlino, S. On the 2,5-Diazolic Heterocyclic System. I. Crystal and Molecular Structure of 3,4-Diphenyl-1,2,5-selenadiazole and 3,4-Diphenyl-1,2,5-thiadiazole. Acta. Cryst. B 1976, 32, 1074–1078. [Google Scholar] [CrossRef]
- Katayama, C.; Honda, M.; Kumagai, H.; Tanaka, J.; Saito, G.; Inokuchi, H. Crystal Structures of Complexes between Hexacyanobutadiene and Tetramethyltetrathiafulvalene and Tetramethylthio-tetrathiafulvalene. Bull. Chem. Soc. Jpn. 1985, 58, 2272–2278. [Google Scholar]
- Guionneau, P.; Chasseau, D.; Howard, J.A.K.; Day, P. Neutral Bis(ethylenedithio)tetrathiaful-valene at 100 K. Acta. Cryst. C 2000, 56, 453–454. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta. Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta. Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Sayle, R.; Milner-White, E.J. RASMOL: Biomolecular Graphics for All. Trends. Biochem. Sci. 1995, 20, 374–376. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; Dapprich, S.; Millam, J.M.; Daniels, A.D.; Kudin, K.N.; Strain, M.C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G.A.; Ayala, P.Y.; Cui, Q.; Morokuma, K.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Cioslowski, J.; Ortiz, J.V.; Baboul, A.G.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Andres, J.L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E.S.; Pople, J.A. Gaussian 98, Revision A7; Gaussian Inc.: Pittsburgh, PA, USA, 1998. [Google Scholar]
- Mizutani, F. PGV; Institute for Molecular Science: Okazaki, Japan, 1996. Available online: http://ccinfo.ims.ac.jp/pgv/pgv_eg.htm.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tomura, M.; Yamashita, Y. An Unsymmetrical Tetrathiafulvalene with a Fused 1,2,5-Thiadiazole Ring and Methylthio Groups. Molecules 2009, 14, 4266-4274. https://doi.org/10.3390/molecules14104266
Tomura M, Yamashita Y. An Unsymmetrical Tetrathiafulvalene with a Fused 1,2,5-Thiadiazole Ring and Methylthio Groups. Molecules. 2009; 14(10):4266-4274. https://doi.org/10.3390/molecules14104266
Chicago/Turabian StyleTomura, Masaaki, and Yoshiro Yamashita. 2009. "An Unsymmetrical Tetrathiafulvalene with a Fused 1,2,5-Thiadiazole Ring and Methylthio Groups" Molecules 14, no. 10: 4266-4274. https://doi.org/10.3390/molecules14104266
APA StyleTomura, M., & Yamashita, Y. (2009). An Unsymmetrical Tetrathiafulvalene with a Fused 1,2,5-Thiadiazole Ring and Methylthio Groups. Molecules, 14(10), 4266-4274. https://doi.org/10.3390/molecules14104266