18-Crown-6 and Dibenzo-18-crown-6 Assisted Extraction of Cesium from Water into Room Temperature Ionic Liquids and Its Correlation with Stability Constants for Cesium Complexes
Abstract
:1. Introduction
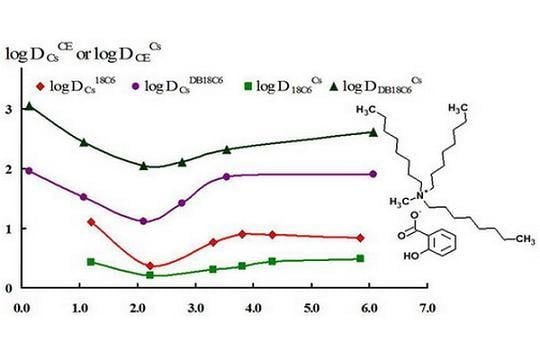
2. Results and Discussion
3. Experimental
3.1. General
3.2. Synthesis and characterization of the RTILs
3.3. Extraction
4. Conclusions
Acknowledgements
References and Notes
- Takeda, Y.; Kawarabayashi, A.; Endō, K.; Yahata, T.; Kudo, Y.; Katsuta, S. Solvent extraction of alkali metal (Li–Cs) picrates with 18-crown-6 into various diluents. Elucidation of fundamental equilibria which govern the extraction-ability and -selectivity. Anal. Sci. 1998, 14, 215–223. [Google Scholar] [CrossRef]
- Levitskaia, T.G.; Maya, L.; van Berkel, G.J.; Moyer, B.A. Anion Partitioning and ion-pairing behavior of anions in the extraction of cesium salts by 4,5‘‘-bis(tert-octylbenzo)dibenzo-24- crown-8 in 1,2-dichloroethane. Inorg. Chem. 2007, 46, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.A. Extraction of cations by crown-ethers. Moscow Univ. Chem. Bull. 2000, 41, 3–15. [Google Scholar]
- Robak, W.; Apostoluk, W.; Maciejewski, P. Analysis of liquid–liquid distribution constants of nonionizable crown ethers and their derivatives. Anal. Chim. Acta 2006, 569, 119–131. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Ansari, S.A.; Sarkar, A.; Bhattacharyya, A.; Manchanda, V.K. Evaluation of calix-crown ionophores for selective separation of radio-cesium from acidic nuclear waste solution. Anal. Chim. Acta 2006, 571, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Cocalia, V.A.; Holbrey, J.D.; Gutowski, K.E.; Bridges, N.J.; Rogers, R.D. Separations of metal ions using ionic liquids: The challenges of multiple mechanisms. Tsinghua Sci. Technol. 2006, 11, 188–193. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Barnes, C.E. Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J. Chem. Soc., Dalton Trans. 1999, 1201–1202. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Griffin, S.T.; Rogers, R.D. Traditional extractants in nontraditional solvents: Groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2000, 39, 3596–3604. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.; Bonnesen, P.V.; Buchanan, A.C., III. Separation of fission products based on ionic liquids: Task-specific ionic liquids containing an aza-crown ether fragment. J. Alloys Compounds 2006, 418, 195–199. [Google Scholar] [CrossRef]
- Chen, P.Y. The assessment of removing strontium and cesium cations from aqueous solutions based on the combined methods of ionic liquid extraction and electrodeposition. Electrochim. Acta 2007, 52, 5484–5492. [Google Scholar] [CrossRef]
- Chun, S.; Dzyuba, S.V.; Bartsch, R.A. Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether. Anal. Chem. 2001, 73, 3737–3741. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.E.; Rogers, R.D. Room-temperature ionic liquids: New solvents for f-element separations and associated solution chemistry. J. Solid State Chem. 2003, 171, 109–113. [Google Scholar] [CrossRef]
- Shimojo, K.; Goto, M. Solvent Extraction and stripping of silver ions in room-temperature ionic liquids containing calixarenes. Anal. Chem. 2004, 76, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H., Jr.; Rogers, R.D. Task-Specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: synthesis, characterization, and extraction studies. Environ. Sci. Technol. 2002, 36, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H., Jr.; Rogers, R.D. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 135–136. [Google Scholar] [CrossRef]
- Ajioka, T.; Oshima, S.; Hirayama, N. Use of 8-sulfonamidoquinoline derivatives as chelate extraction reagents in ionic liquid extraction system. Talanta 2008, 74, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Lohithakshan, K.V.; Aggarwal, S.K. Solvent extraction studies of Pu(IV) with CMPO in 1-octyl- 3-methylimidazolium hexafluorophosphate (C8mimPF6) room temperature ionic liquid (RTIL). Radiochim. Acta 2008, 96, 93–97. [Google Scholar] [CrossRef]
- Dietz, M.L.; Stepinski, D.C. Anion concentration-dependent partitioning mechanism in the extraction of uranium into room-temperature ionic liquids. Talanta 2008, 75, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Nishi, N.; Murakami, H.; Imakura, S.; Kakiuchi, T. Facilitated transfer of alkali-metal cations by dibenzo-18-crown-6 across the electrochemically polarized interface between an aqueous solution and a hydrophobic room-temperature ionic liquid. Anal. Chem. 2006, 78, 5805–5812. [Google Scholar] [CrossRef] [PubMed]
- Vendilo, A.G.; Djigailo, D.I.; Rönkkömäki, H.; Lajunen, M.; Chernikova, E.A.; Lajunen, L.H.J.; Pletnev, I.V.; Popov, K.I. Thermodynamics of cesium complexes formation with 18-crown-6 in hydrophobic ionic liquids. A correlation with extraction capability. In Macrocyclic Chemistry: New Research Developments, 1st ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2010; in press. [Google Scholar]
- Yakshin, V.V.; Vilkova, O.N.; Tsarenko, N.A.; Demin, S.V.; Zhilov, V.I.; Tsivadze, A.Y. Molecular Design of macrocyclic extractants for extraction and separation of alkali and alkaline- earth metals. Russ. J. Coord. Chem. 2006, 32, 83–87. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sakamoto, Y. Complex formation of alkali metal ions with 18-crown-6 and its derivatives in 1,2-dichloroethane. Anal. Chim. Acta 2000, 403, 325–332. [Google Scholar] [CrossRef]
- Arnaud-Neu, F.; Delgado, R.; Chaves, S. Critical evaluation of stability constants and thermodynamic functions of metal complexes of crown ethers. Pure Appl. Chem. 2003, 75, 71–102. [Google Scholar] [CrossRef]
- IUPAC Stability Constants Database, version 2007; Academic Software: Timble, UK.
- Egorov, V.M.; Smirnova, S.V.; Pletnev, I.V. Highly efficient extraction of phenols and aromatic amines into novel ionic liquids incorporating quaternary ammonium cation. Sep. Purif. Technol. 2008, 63, 710–715. [Google Scholar] [CrossRef]
- Vilkova, O.M.; Yakshin, V.V. Extraction-photometric determination of the composition of multicomponent crown ether—Podand mixtures. J. Anal. Chem. 2003, 58, 27–30. [Google Scholar] [CrossRef]
Sample Availability: 18C6, DB18C6, [BMIM][PF6], [BMIM][N(Tf)2], and [HMIM][N(Tf)2] are commercially available. |






| RTIL | Vo, mL | Vw, mL | Aqueous pH | logDCs |
|---|---|---|---|---|
| [THA][DHSS] | 0.5 | 5.0 | 5.94 | 1.21 |
| 3.36 | 1.11 | |||
| 1.59 | 0.84 | |||
| [TOMA][Sal] | 0.5 | 5.0 | 5.98 | 0.69 |
| 4.95 | 0.76 | |||
| 3.01 | 0.74 | |||
| 1.13 | 0.10 | |||
| [BMIM][PF6] | 0.5 | 5.0 | 5.90 | -0.59 |
| 3.33 | -0.82 | |||
| 1.25 | -0.80 | |||
| [BMIM][N(Tf)2] | 1.0 | 3.0 | 6.44 | -0.67 |
| 3.35 | -1.24 | |||
| 1.25 | -1.31 | |||
| [EtHMIM][N(Tf)2] | 1.0 | 3.0 | 6.34 | -0.81 |
| 2.09 | -0.62 | |||
| [HMIM][N(Tf)2] | 2.0 | 2.0 | 5.82 | -1.24 |
| 2.84 | -1.36 | |||
| 0.93 | -2.21 |
| RTIL | Aqueous pH | logDCs18C6 | logD18C6Cs |
|---|---|---|---|
| [THA][DHSS] | 5.92 | 0.24 | -0.12 |
| 4.30 | 0.23 | -0.16 | |
| 3.86 | 0.03 | -0.41 | |
| 3.39 | -0.38 | -0.50 | |
| 2.48 | 0.57 | -0.15 | |
| 1.60 | 0.75 | -0.01 | |
| [TOMA][Sal] | 5.84 | 0.83 | 0.48 |
| 4.33 | 0.89 | 0.44 | |
| 3.81 | 0.90 | 0.36 | |
| 3.31 | 0.77 | 0.30 | |
| 2.22 | 0.36 | 0.21 | |
| 1.20 | 1.11 | 0.43 | |
| [BMIM][PF6] | 5.84 | -0.20 | 0.13 |
| 4.33 | -0.45 | 0.08 | |
| 3.81 | -0.62 | 0.06 | |
| 3.31 | -1.27 | 0.00 | |
| 2.22 | -0.74 | 0.00 | |
| 1.20 | -0.66 | 0.08 | |
| [BMIM][N(Tf)2] | 6.88 | 1.56 | 0.77 |
| 5.60 | 1.43 | 0.65 | |
| 3.56 | -0.26 | 0.50 | |
| 2.26 | -0.27 | 0.08 | |
| 1.27 | -0.23 | 0.23 | |
| [EtHMIM][N(Tf)2] | 6.50 | 0.56 | -0.27 |
| 2.15 | 0.30 | -0.45 | |
| [HMIM][N(Tf)2] | 5.60 | 0.82 | 0.25 |
| 4.10 | 0.91 | 0.17 | |
| 2.96 | 0.61 | 0.06 | |
| 1.40 | -0.22 | 0.08 | |
| 0.90 | -0.33 | 0.27 |
| RTIL | Cw0(Cs), mol·dm-3 | Co0(18C6), mol·dm-3 | Aqueous pH | logDCsDB18C6 | logDDB18C6Cs |
|---|---|---|---|---|---|
| [THA][DHSS] | 5.0·10-4 | 5.0·10-2 | 6.03 | 1.56 | 2.68 |
| 2.85 | 1.27 | 2.41 | |||
| 2.45 | 0.89 | 2.20 | |||
| 1.89 | 0.74 | 2.15 | |||
| 1.06 | 0.86 | 2.30 | |||
| 0.14 | 1.02 | 2.68 | |||
| [TOMA][Sal] | 5.0·10-4 | 5.0·10-2 | 6.07 | 1.91 | 2.62 |
| 3.54 | 1.86 | 2.32 | |||
| 2.77 | 1.42 | 2.11 | |||
| 2.11 | 1.12 | 2.05 | |||
| 1.08 | 1.52 | 2.45 | |||
| 0.13 | 1.96 | 3.05 | |||
| [BMIM][N(Tf)2] | 1.5·10-3 | 1.5·10-1 | 6.93 | 2.28 | 2.89 |
| 5.09 | 2.06 | 2.71 | |||
| 3.16 | 0.14 | 2.57 | |||
| 1.92 | -0.47 | 2.63 | |||
| 0.38 | -0.60 | 2.94 |
| Solvent | logK1 | logK2 | logDCs | logDCs18C6 | logD18C6Cs | log[DCs18C6/(DCsD18C6Cs)] |
|---|---|---|---|---|---|---|
| [HMIM][N(Tf)2] | 4.4 | 1.13 | −1.24 | 0.82 | 0.25 | 1.81 |
| [EtHMIM][N(Tf)2] | 3.4 | 1.16 | −0.81 | 0.56 | −0.27 | 1.64 |
| [BMIM][N(Tf)2] | 3.4 | 1.29 | −0.67 | 1.56 | 0.77 | 1.46 |
| [BMIM][PF6] | 2.3 | −0.59 −0.59b −1.17c | −0.20 −0.52d | 0.13 0.15e | 0.26 | |
| [TOMA][Sal] | 1.45 | 0.69 | 0.83 | 0.49 | −0.38 | |
| [THA][DHSS] | 0.77 | 1.21 | 0.25 | −0.12 | −0.84 | |
| 1,2-dichloroethane | 7.98f | 2.58f | −7.7g | 0.8h | 0.03i | 6.93 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vendilo, A.G.; Djigailo, D.I.; Smirnova, S.V.; Torocheshnikova, I.I.; Popov, K.I.; Krasovsky, V.G.; Pletnev, I.V. 18-Crown-6 and Dibenzo-18-crown-6 Assisted Extraction of Cesium from Water into Room Temperature Ionic Liquids and Its Correlation with Stability Constants for Cesium Complexes. Molecules 2009, 14, 5001-5016. https://doi.org/10.3390/molecules14125001
Vendilo AG, Djigailo DI, Smirnova SV, Torocheshnikova II, Popov KI, Krasovsky VG, Pletnev IV. 18-Crown-6 and Dibenzo-18-crown-6 Assisted Extraction of Cesium from Water into Room Temperature Ionic Liquids and Its Correlation with Stability Constants for Cesium Complexes. Molecules. 2009; 14(12):5001-5016. https://doi.org/10.3390/molecules14125001
Chicago/Turabian StyleVendilo, Andrey Grigoryevich, Dmitry Ivanovich Djigailo, Svetlana Valeryevna Smirnova, Irina Ivanovna Torocheshnikova, Konstantin Ivanovich Popov, Vladimir Georgyevich Krasovsky, and Igor Vladimirovich Pletnev. 2009. "18-Crown-6 and Dibenzo-18-crown-6 Assisted Extraction of Cesium from Water into Room Temperature Ionic Liquids and Its Correlation with Stability Constants for Cesium Complexes" Molecules 14, no. 12: 5001-5016. https://doi.org/10.3390/molecules14125001
APA StyleVendilo, A. G., Djigailo, D. I., Smirnova, S. V., Torocheshnikova, I. I., Popov, K. I., Krasovsky, V. G., & Pletnev, I. V. (2009). 18-Crown-6 and Dibenzo-18-crown-6 Assisted Extraction of Cesium from Water into Room Temperature Ionic Liquids and Its Correlation with Stability Constants for Cesium Complexes. Molecules, 14(12), 5001-5016. https://doi.org/10.3390/molecules14125001