Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics
Abstract
:1. Introduction

2. Result and Discussion
2.1. The χc change of MCC analyzed by XRD


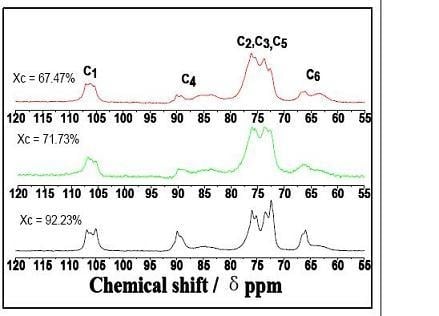
2.2. CP/MAS 13C-NMR Analysis
| Carbon atom | Chemical shift ( ppm, δ) | ||
|---|---|---|---|
| Xc = 92.23% | Xc = 71.73% | Xc = 67.47% | |
| C1 | 106.7,106, 105 | 106.8,106.2,105.3 | 106.8,105.9,105.3 |
| Crystalline C4 | 89.9 | 89.9 | 90 |
| Amorphous C4 | 84.6 | 83.7 | 83.6 |
| C2, C3, C5 | 75.8, 73.4, 72.3 | 76, 75.5, 73.7 | 76.1, 73.7, 72.6 |
| Crystalline C6 | 65.9 | 66.5 | 66.1 |
| Amorphous C6 | 63.5 | 64.8 | 63.3 |


| Xc, % | Chemical shift Assignments | Chemical shift ppm | Intensity % |
|---|---|---|---|
| 92.23 | Іα | 90.3 | 12.6 |
| І (α+β) | 89.8 | 19.6 | |
| Para-crystalline | 89.4 | 17.3 | |
| Іβ | 88.9 | 19.0 | |
| Amorphous | 86.5 | 8.2 | |
| Fibril surface | 85.1 | 11.1 | |
| Fibril surface | 83.6 | 12.2 | |
| 71.73 | Іα | 89.7 | 5.2 |
| І (α+β) | 89.9 | 13.8 | |
| Para-crystalline | 88.9 | 16.0 | |
| Іβ | 88.1 | 13.1 | |
| Amorphous | 86 | 13.5 | |
| Fibril surface | 85 | 15.3 | |
| Fibril surface | 83.5 | 23.1 | |
| 67.47 | Іα | 90.7 | 1.3 |
| І (α+β) | 90.1 | 9.8 | |
| Para-crystalline | 89.4 | 13.3 | |
| Іβ | 89 | 12.3 | |
| Amorphous | 86.2 | 20.6 | |
| Fibril surface | 84.9 | 19.9 | |
| Fibril surface | 83.4 | 22.8 |

| Xc, % | Chemical shift Assignments | Crystalline C6 | Amorphous C6 |
|---|---|---|---|
| 92.23 | Chemical shift (ppm) | 66.6,65.9 | 64.8,63.4,62 |
| Intensity (%) | 21.4,38 | 10.8,20.4,9.4 | |
| Total Intensity (%) | 59.4 | 40.6 | |
| 71.73 | Chemical shift (ppm) | 66.8,65.9 | 65.1,63.3,60.7 |
| Intensity (%) | 22.7,12.7 | 11.3,22.3,30.9 | |
| Total Intensity (%) | 35.4 | 64.6 | |
| 67.47 | Chemical shift (ppm) | 66.9,66.1 | 64.5,63.3,62.1 |
| Intensity (%) | 13.4,18.9 | 23.7,29.3,14.6 | |
| Total Intensity (%) | 32.4 | 67.6 |
2.3. XPS analysis of MCC treated with phosphoric acid


| Xc % | Peak position | Separation | Area | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EB/eV | EB/eV | A/% | |||||||
| C1 | C2 | C3 | C1 | C2 | C3 | C1 | C2 | C3 | |
| 92.23 | 283.00 | 284.77 | 286.23 | 0.00 | 1.77 | 3.23 | 14.5 | 68.1 | 16.4 |
| 71.73 | 283.42 | 284.70 | 286.00 | 0.00 | 1.28 | 2.58 | 28.2 | 58.9 | 12.9 |
| 67.47 | 283.40 | 284.66 | 285.73 | 0.00 | 1.26 | 2.33 | 33.1 | 56.3 | 10.6 |
2.4. The kinetic analysis of cellulose dissolution in phosphoric acid






| Parameters | 30 °C | 50 °C | 70 °C |
|---|---|---|---|
| K/h-1 | 0.06 | 0.17 | 0.12 |
| A0/h-1 | 1.2 × 106 | ||
| Ea/KJ·mol-1 | 42.4 | ||


3. Experimental
3.1. Materials
3.2. Sample preparation
3.3. X-ray diffraction method (XRD)

3.4. Solid-State Cross-Polarization Magic Angle Spinning (CP/MAS) 13C-NMR
3.5. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Acknowledgements
References
- Jarvis, M. Cellulose stacks up. Nature 2003, 426, 611–612. [Google Scholar] [CrossRef]
- Sianott, M.L. The cellobiohydrolases of Trichoderma reesei: A review of indirect and direct evidence that their function is not just glycosidic bond hydrolysis. Biochem. Soc. Trans. 1998, 26, 160–164. [Google Scholar]
- Laser, M.; Schulman, D.; Allen, S.G.; Lichwa, J.; Antal, M.J.; Lynd, L.R. A comparision of liquid hot water and steam pretreatment of sugar cane baggage for bioconversion to ethanol. Biores. Technol. 2002, 81, 33–44. [Google Scholar] [CrossRef]
- Ooshima, H.; Burns, D.S.; Converse, A.O. Adsorption of cellulase from Trichoderma reesei on cellulose and lignacious residue in wood pretreated by dilute sulfuric acid with explosive decompression. Biotechnol. Bioeng. 1990, 36, 446–453. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.; Yu, H.; Zhang, B.; Jin, F. Purification and characterization of piceid-B-D-glucosidase from Aspergillusoryzae. Process Biochem. 2006, 42, 83–88. [Google Scholar]
- Mawadza, C.; Hatti-Kual, R.; Zvamya, R.; Mattiasson, B. Purificaation and characterization of cellulases produced by two Bacilus strains. J. Biotechnol. 2000, 83, 177–187. [Google Scholar]
- Dadi, A.P.; Varanasi, S.; Schall, C.A. Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol. Bioeng. 2006, 95, 904–910. [Google Scholar] [CrossRef]
- Kwan, C.C.; Ghadiri, M.; Papadopoulos, D.G.; Bentham, A.C. The effects of operating conditions on the milling of microcrystalline cellulose. Chem. Eng. Technol. 2003, 26, 185–190. [Google Scholar] [CrossRef]
- Sun, R.C.; Mott, L.; Bolton, J. Isolation and fractional characterization of ball-milled and enzyme lignins from oil palm trunk. J. Agric. Food Chem. 1998, 46, 718–723. [Google Scholar] [CrossRef]
- Sun, X.F.; Sun, R.C.; Paul, F.; Baird, M.S. Extraction and characterization of original lignin and hemicelluloses from MCC. J. Agric. Food Chem. 2005, 53, 860–870. [Google Scholar]
- Zhao, H.; Kwak, J.H.; Yong, W.; Franz, J.A.; White, J.M. Effects of crystallinity on dilute acid hydrolysis of cellulose by cellulose ball-milling study. Energy Fuels 2006, 20, 807–811. [Google Scholar] [CrossRef]
- Anantharam, P.D.; Sasidhar, V.; Constange, A.S. Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol. Bioeng. 2006, 95, 904–910. [Google Scholar] [CrossRef]
- Roseneau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and by-product formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Tsygankova, N.G.; Grinshpan, D.D.; Koren, A.O. Mndo modeling of complex formation in N,N-dimethylacetamide-lithium chloride cellulose dissolving system. Cell. Chem. Technol. 1996, 30, 357–373. [Google Scholar]
- Wei, S.; Kumar, V.; Banker, G.S. Phosphoric acid mediated depolymerization and decrystallization of cellulose: preparation of low crystallinity cellulose-A new pharmaceutical excipient. Int. J. Pharm. 1996, 142, 175–181. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Cui, J.; Lynd, L.R.; Kuang, L.R. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 2006, 7, 644–648. [Google Scholar] [CrossRef]
- Whitmore, R.E.; Atalla, R.H. Factors influencing the regeneration of cellulose I from phosphoric acid. Int. Biol. Macromol. 1985, 7, 182. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Ding, S.Y.; Mielenz, J.R.; Cui, J.B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioen. 2007, 97, 214–223. [Google Scholar]
- Mosier, N.; Wyman, C.; Dalem, B. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Walseth, C.S. Occurrence of Cellulases in enzyme preparations from microorganisms. Tappi 1952, 35, 228–233. [Google Scholar]
- Zhang, Y.H.P.; Lynd, L.R. Determination of the number average degree of polymerization of cellodextrins and cellulose with application to enzymatic hydrolysis. Biomacromolecules 2005, 6, 1510–1515. [Google Scholar] [CrossRef]
- Ekenstam, A. Fractional solution of rayon pulp in phosphoric acid; a new method for studying the polymerization. Svensk Papperstidn. 1942, 45, 61. [Google Scholar]
- Danilove, S.N.; Gintse, N.F. The role of phosphoric acid in the study and treatment of cellulose. I swelling and solution of cellulose in phosphoric acid. Z.h. Obsch. Khim. 1956, 26, 3014. [Google Scholar]
- Teeäär, R.; Lippmaa, E. Solid state carbon-13 NMR of cellulose: Relaxation study. Polym. Bull. 1984, 12, 315–318. [Google Scholar] [CrossRef]
- Kono, H.; Yunoki, S.; Shikano, T.; Fujiwara, M.; Erata, T.; Takai, M. CP/MAS 13C NMR study of cellulose and cellulose derivatives. 1. Complete assignment of the CP/MAS 13C NMR spectrum of the native cellulose. J. Am. Chem. Soc. 2002, 124, 7506–7511. [Google Scholar] [CrossRef]
- Bardet, M.; Emsley, L.; Vincendon, M. Two-dimensional spin-exchange solid-state NMR studies of 13C-enriched wood. Solid State Nucl. Magn. Reson. 1997, 8, 25–32. [Google Scholar]
- Morjanoff, P.J.; Gray, P.P. Optimization of steam explosion as a method for increasing susceptibility of sugarcane bagasse to enzymatic saccharification. Biotechnol. Bioeng. 1987, 29, 733–741. [Google Scholar] [CrossRef]
- Galas, E.P; Romanowskia, Y.R. Hydrolysis and transformation of cellulose with aspergillus niger IBT-90 enzymes. Acta Biotechnol. 1997, 17, 339–349. [Google Scholar]
- Larsson, P.T.; Wickholm, K.; Iversen, T.A. CP/MAS13C NMR investigation of molecular ordering in celluloses. Carbohyd. Res. 1997, 302, 19–25. [Google Scholar] [CrossRef]
- Focher, B.; Palma, M.T.; Canetti, M.; Torri, G.; Cosentino, C.; Gastaldi, G. Structural differences between non-wood plant celluloses: Evidence from solid NMR, vibrational spectroscopy and X-ray diffractometry. Ind. Crops Prod. 2001, 13, 193–208. [Google Scholar] [CrossRef]
- Heux, L.; Dinand, E.; Vignon, M.R. Structural aspects in ultrathin cellulose microfibrils followed by 13C CP-MAS NMR. Carbohyd. Polym. 1999, 40, 115–124. [Google Scholar] [CrossRef]
- Dorris, G.M.; Gray, D.G. The surface analysis of paper and wood fibres by ESCA. II. Surface composition of mechanical pulps. Cell. Chem. Technol. 1978, 12, 721–734. [Google Scholar]
- Eken-Saracoglu, N.; Mutlu, S.F.; Dilmac, G.; Cavusoglu, H. A comparative kinetic study of acidic hemicellulose hydrolysis in corn cob and sunflower seed hull. Biores. Technol. 1998, 65, 29–33. [Google Scholar] [CrossRef]
- Qi, H.S.; Chang, C.Y.; Zhang, L.N. Effects of temperature and molecular weight on dissolution of cellulose in NaOH/urea aqueous solution. Cellulose 2008, 15, 779–787. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.N.; Guo, S.L. Mechanisms of lead biosorption on cellulose/chitin beads. Water Res. 2005, 39, 3755–3762. [Google Scholar]
- Focher, B.; Palma, M.; Canetti, T.; Torri, M.; Cosentino, G.C.; Gastaldi, G. Structural differences between non-wood plant celluloses: Evidence from solid state NMR, vibrational spectroscopy and X-ray diffractometry. Ind. Crops Products 2001, 13, 193–208. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Zhang, J.; Lin, L.; Chen, T.; Zhang, J.; Liu, S.; Li, Z.; Ouyang, P. Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics. Molecules 2009, 14, 5027-5041. https://doi.org/10.3390/molecules14125027
Zhang J, Zhang J, Lin L, Chen T, Zhang J, Liu S, Li Z, Ouyang P. Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics. Molecules. 2009; 14(12):5027-5041. https://doi.org/10.3390/molecules14125027
Chicago/Turabian StyleZhang, Junhua, Jingqiang Zhang, Lu Lin, Tianming Chen, Jun Zhang, Shijie Liu, Zhenjiang Li, and Pingkai Ouyang. 2009. "Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics" Molecules 14, no. 12: 5027-5041. https://doi.org/10.3390/molecules14125027
APA StyleZhang, J., Zhang, J., Lin, L., Chen, T., Zhang, J., Liu, S., Li, Z., & Ouyang, P. (2009). Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics. Molecules, 14(12), 5027-5041. https://doi.org/10.3390/molecules14125027