Methyl Carbonium Ion Migration during the Reaction of 4-Chloro-5-methoxyl-3(2H)-pyridazinone with Trifluoroethylation Agents
Abstract
:Introduction

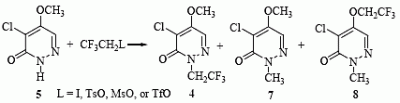
Results and Discussion

| Entry | Condition | Product (yield) | Time | |
|---|---|---|---|---|
| 1 | ICH2CF3 | K2CO3/DMF, 80 °C | 4 (5%), 7 (20%) | 12 h |
| 2 | TsOCH2CF3 | K2CO3/DMF, 120 °C | No product | 20 h |
| 3 | MsOCH2CF3 | K2CO3/18-crown-6/DMF, 120 °C | 7 (40%) | 20 h |
| 4 | MsOCH2CF3 | K2CO3/18-crown-6/1,4-Dioxane, reflux | 4 (trace), 7 (35%) | 20 h |
| 5 | MsOCH2CF3 | K2CO3/18-crown-6/HMPA, 140 °C | 8 (45%) | 20 h |
| 6 | MsOCH2CF3 | NaH/18-crown-6/HMPA, 140 °C | 4 (15%), 8 (25%) | 20 h |
| 7 | TfOCH2CF3 | K2CO3/18-crown-6/HMPA, 60 °C | 4 (40%), 7 (25%) | 24 h |




Conclusions
Experimental
General
General procedure for the synthesis of compound 4
Acknowledgements
References and Notes
- Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Chambers, R.D. Fluorine in Organic Chemistry; Blackwell: Cambridge, MA, USA, 2004. [Google Scholar]
- Ismail, F.M.D. Important fluorinated drugs in experimental and clinical use. J. Fluorine Chem. 2002, 118, 27–33. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 2004, 5, 570–589. [Google Scholar] [CrossRef]
- Quazepam. Merck Index; Merck: New York, USA, 1996; Volume 12, p. 8211. [Google Scholar]
- Halazepamin. Merck Index; Merck: New York, USA, 1996; Volume 12, p. 4619. [Google Scholar]
- Steinmann, M.; Topliss, J.G.; Alekel, R.; Wong, Y.-S.; York, E.E. 1-Poly(fluoroalkyl)benzo- diazepines. J. Med. Chem. 1973, 16, 1354–1360. [Google Scholar] [CrossRef]
- Flecainide. Merck Index; Merck: New York, USA, 1996; Volume 12, p. 4136. [Google Scholar]
- Banitt, E.H.; Schmid, J.R.; Mendel, A. Antiarrhythmics. N-(Aminoalkylene)trifluoroethoxy-benzamides and N-(aminoalkylene)trifluoroethoxynaphthamides. J. Med. Chem. 1975, 18, 1130–1134. [Google Scholar] [CrossRef]
- Banitt, E.H.; Bronn, W.R.; Coyne, W.E.; Schmid, J.R. Antiarrhythmics. 2. Synthesis and antiarrhythmic activity of N-(piperidylalkyl)trifluoroethoxybenzamides. J. Med. Chem. 1977, 20, 821–826. [Google Scholar] [CrossRef]
- Maes, B.U.W.; R’kyek, O.; Košmrlj, J.; Lemière, G.L.F.; Esmans, E.; Rozenski, J.; Dommisse, R.A.; Haemers, A. Suzuki reactions on chloropyridazinones: an easy approach towards arylated 3(2H)-pyridazinones. Tetrahedron 2001, 57, 1323–1330. [Google Scholar] [CrossRef]
- Riedl, Z.; Maes, B.U.W.; Monsieurs, K.; Lemiere, G.L.F.; Matyus, P.; Hajos, G. Synthesis of new pyridazino[4,5-c]isoquinolinones by Suzuki cross-coupling reaction. Tetrahedron 2002, 58, 5645–5650. [Google Scholar] [CrossRef]
- Frank, H.; Heinisch, G. Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1990; Vol. 27, pp. 1–49. [Google Scholar]
- Rkyek, O.; Maes, B.U.W.; Jonckers, T.H.M.; Lemiere, G.L.F.; Dommisse, R. A. New enediyne derivatives: synthesis of symmetrically and unsymmetrically disubstituted 4,5-dialkynyl-3(2H)-pyridazinones. Tetrahedron 2001, 57, 10009–10016. [Google Scholar] [CrossRef]
- Dury, K. New methods in the chemistry pyridazones. Angew. Chem. Int. Ed. Engl. 1965, 4, 292–300. [Google Scholar] [CrossRef]
- Lyga, J.W. The reaction of 2-substituted-4,5-dihalo-3(2H)-pyridazinones with alkoxides and alkylthiolates. J. Heterocyclic Chem. 1988, 25, 1757–1760. [Google Scholar] [CrossRef]
- Cho, S-D; Choi, W-Y; Yoon, Y.-J. Concurrent alkylation-methoxylation of 4,5-dihalopyridazin-6-ones and synthesis of 5-halo-4-hydroxypyridazin-6-ones. J. Heterocyclic Chem. 1996, 33, 1579–1582. [Google Scholar] [CrossRef]
- David, T.M. Mucochloric acid. II. Reactions of the aldehyde group. J. Am. Chem. Soc. 1953, 75, 1909–1910. [Google Scholar] [CrossRef]
- Hine, J.; Ghirardelli, R.G. The SN- reactivity of β-fluoroethyl iodides. J. Org. Chem. 1958, 23, 1550–1552. [Google Scholar] [CrossRef]
- Quinn, K.J.; Biddick, N.A.; De Christopher, B. A. Ring expansion of trans-divinyl ethylene oxide by oxonium ylide [2,3] sigmatropic rearrangement. Tetrahedron Lett. 2006, 47, 7281–7283. [Google Scholar]
- Sample Availability: Samples of the compounds 4, 6, 8 are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Q.; Lin, G.; Liu, L.; Yang, Z.; Zhang, L.-H. Methyl Carbonium Ion Migration during the Reaction of 4-Chloro-5-methoxyl-3(2H)-pyridazinone with Trifluoroethylation Agents. Molecules 2009, 14, 777-784. https://doi.org/10.3390/molecules14020777
Li Q, Lin G, Liu L, Yang Z, Zhang L-H. Methyl Carbonium Ion Migration during the Reaction of 4-Chloro-5-methoxyl-3(2H)-pyridazinone with Trifluoroethylation Agents. Molecules. 2009; 14(2):777-784. https://doi.org/10.3390/molecules14020777
Chicago/Turabian StyleLi, Qin, Guichun Lin, Li Liu, Zhenjun Yang, and Li-He Zhang. 2009. "Methyl Carbonium Ion Migration during the Reaction of 4-Chloro-5-methoxyl-3(2H)-pyridazinone with Trifluoroethylation Agents" Molecules 14, no. 2: 777-784. https://doi.org/10.3390/molecules14020777
APA StyleLi, Q., Lin, G., Liu, L., Yang, Z., & Zhang, L. -H. (2009). Methyl Carbonium Ion Migration during the Reaction of 4-Chloro-5-methoxyl-3(2H)-pyridazinone with Trifluoroethylation Agents. Molecules, 14(2), 777-784. https://doi.org/10.3390/molecules14020777