Phosphotungstic Acid: An Efficient, Cost-effective and Recyclable Catalyst for the Synthesis of Polysubstituted Quinolines
Abstract
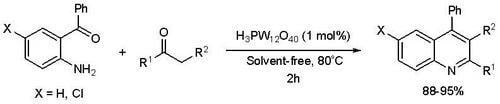
:Introduction
Results and Discussion

| Entrya | Catalyst (mol%) | Yield (%)b | Time (h) |
|---|---|---|---|
| 1 | H3PW12O40 (0.1) | 30 | 24 |
| 2 | H3PW12O40 (0.2) | 45 | 20 |
| 3 | H3PW12O40 (0.3) | 50 | 12 |
| 4 | H3PW12O40 (0.4) | 50 | 8 |
| 5 | H3PW12O40 (0.5) | 50 | 6 |
| 6 | H3PW12O40 (0.6) | 55 | 5 |
| 7 | H3PW12O40 (0.7) | 60 | 3.5 |
| 8 | H3PW12O40 (0.8) | 75 | 3 |
| 9 | H3PW12O40 (0.9) | 83 | 2.5 |
| 10 | H3PW12O40 (1) | 90 | 2 |
| 11 | H3PW12O40 (1.1) | 91 | 2 |
| 12 | H3PW12O40 (1.2) | 91 | 2 |
| 13 | HClO4 (1) | 40 | 12 |
| 14 | H2SO4 (1) | 30 | 12 |
| 15 | SSA (1) | 60 | 5 |
| 16 | ZnCl2 (1) | 40 | 12 |
| 17 | TsOH (1) | 50 | 12 |
| Entry | 2-Aminoaryl ketone | CH-acid | Product | Yield (%)a | Mp (˚C) Found | Mp (˚C) Reported |
| 1 |  |  |  | 90 | 102-103 | 100-101b |
| 2 |  |  |  | 92 | 110-112 | 111-112b |
| 3 |  |  |  | 94 | 153-154 | 156-157c |
| 4 |  |  |  | 90 | 130-131 | 130-132c |
| 5 |  |  |  | 89 | 192-194 | 190-192c |
| 6 |  |  |  | 92 | 155-157 | 155-156c |
| 7 |  |  |  | 93 | 99-100 | 98-100d |
| 8 |  |  |  | 95 | 152-154 | 153-154e |
| 9 |  |  |  | 94 | 103-105 | 105-107f |
| 10 |  |  |  | 95 | 164-166 | 164-165c |
| 11 |  |  |  | 91 | 150-152 | 150-151c |
| 12 |  |  |  | 89 | 186-187 | 185-186c |
| 13 |  |  |  | 88 | 106-108 | 106-107b |
| 14 |  |  |  | 92 | 207-209 | 208-209c |
| 15 |  |  |  | 94 | 132-134 | 133-135b |
| Run No. | Yield (%)a | Time (h) |
|---|---|---|
| 1 | 93 | 2 |
| 2 | 93 | 2 |
| 3 | 92 | 2.5 |
| 4 | 91 | 2.5 |
| 5 | 92 | 3 |
Experimental
General
General procedure for the synthesis of quinolines in the presence of heteropolyacid
Spectral data for 3g, 3h and 3i
Acknowledgements
References and Notes
- Clark, J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] , and references cited therein.
- Firouzabadi, H.; Jafari, A.A. Heteropoly acids, their salts and polyoxometalates as heterogenous, efficient and eco-friendly catalysts in organic reactions: Some recent advances. J. Iran. Chem. Soc. 2005, 2, 85–114. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Rao, T.S.; Narender, R.; Gupta, M.K. PMA/SiO2 as efficient, cost-effective and recyclable catalytic system for the synthesis of highly substituted pyrroles. J. Mol. Catal. A: Chem. 2007, 278, 42–46. [Google Scholar] [CrossRef]
- Tsukuda, E.; Sato, S.; Takahashi, R.; Sodesawa, T. Production of acrolein from glycerol over silica-supported heteropoly acids. Catal. Commun. 2007, 8, 1349–1353. [Google Scholar] [CrossRef]
- Azizi, N.; Torkian, L.; Saidi, M.R. Highly efficient synthesis of bis(indolyl)methanes in water. J. Mol. Catal. A Chem. 2007, 275, 109–112. [Google Scholar] [CrossRef]
- Azizi, N.; Torkiyan, L.; Saidi, M.R. Highly efficient one-pot three-component Mannich reaction in water catalyzed by heteropoly acids. Org. Lett. 2006, 8, 2079–2082. [Google Scholar]
- Kumar, A.; Singh, P.; Kumar, S.; Chandra, R.; Mozumdar, S. A facile one-pot synthesis of thioethers using heteropoly acids. J. Mol. Catal. A Chem. 2007, 276, 95–101. [Google Scholar] [CrossRef]
- Chen, Y.L.; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and antibacterial evaluation of certain quinolone derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Roma, G.; Braccio, M.D.; Grossi, G.; Chia, M. 1,8-naphthyridines IV. 9-substituted N,N-dialkyl-5-(alkylamino or cycloalkylamino) [1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides, new compounds with anti-aggressive and potent anti-inflammatory activities. Eur. J. Med. Chem. 2000, 35, 1021. [Google Scholar] [CrossRef]
- Billker, O.; Lindo, V.; Panico, M.; Etiene, A.E.; Paxton, T.; Dell, A.; Rogers, M.; Sinden, R.E.; Morris, H.R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 1998, 392, 289–292. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Mendez, L.Y.V.; Gomez, C.M.M. Recent progress in the synthesis of quinolines. Curr. Org. Chem. 2005, 9, 141–161. [Google Scholar] [CrossRef]
- Das, B.; Damodar, K.; Chowdhury, N.; Kumar, R.A. Application of heterogeneous solid acid catalysts for Friedlander synthesis of quinolines. J. Mol. Catal. A Chem. 2007, 274, 148–152. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Salehi, P.; Ghaderi, A.; Shiri, M. A catalytic and green procedure for Friedlander quinoline synthesis in aqueous media. Catal. Commun. 2007, 8, 1214–1218. [Google Scholar] [CrossRef]
- Narasimhulu, M.; Reddy, T.S.; Mahesh, K.C.; Prabhakar, P.; Rao, C.B.; Venkateswarlu, Y. Silica supported perchloric acid: A mild and highly efficient heterogeneous catalyst for the synthesis of poly-substituted quinolines via Friedlander hetero-annulation. J. Mol. Catal. A: Chem. 2007, 266, 114–117. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Salehi, P.; Ghaderi, A.; Shiri, M.; Tanbakouchian, Z. An eco-friendly procedure for the synthesis of polysubstituted quinolines under aqueous media. J. Mol. Catal. A Chem. 2006, 259, 253–258. [Google Scholar]
- Zhang, L.; Wu, J. Friedländer Synthesis of Quinolines Using a Lewis Acid-Surfactant-Combined Catalyst in Water. Adv. Synth. Catal. 2007, 349, 1047–1051. [Google Scholar] [CrossRef]
- Bose, D.S.; Kumar, R.K. An efficient, high yielding protocol for the synthesis of functionalized quinolines via the tandem addition/annulation reaction of o-aminoaryl ketones with alpha-methylene ketones. Tetrahedron Lett. 2006, 47, 813–816. [Google Scholar] [CrossRef]
- Atechian, S.; Nock, N.; Norcross, R.D.; Ratni, H.; Thomas, A.W.; Verron, J.; Masciadri, R. New vistas in quinoline synthesis. Tetrahedron 2007, 63, 2811–2823. [Google Scholar]
- Wang, G.-W.; Jia, C.-S.; Dong, Y.-W. Benign and highly efficient synthesis of quinolines from 2-aminoarylketone or 2-aminoarylaldehyde and carbonyl compounds mediated by hydrochloric acid in water. Tetrahedron Lett. 2006, 47, 1059–1063. [Google Scholar] [CrossRef]
- Yadav, J.S.; Rao, P.P.; Sreenu, D.; Rao, R.S.; Kumar, V.N.; Nagaiah, K.; Prasad, A.R. Sulfamic acid: an efficient, cost-effective and recyclable solid acid catalyst for the Friedlander quinoline synthesis. Tetrahedron Lett. 2005, 46, 7249–7253. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Nikcheh, M.S. Oxalic acid: An efficient and cost-effective organic catalyst for the Friedlander quinoline synthesis under solvent-free conditions. Monatsh. Chem. 2007, 138, 1249–1252. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Sreedhar, P.; Srinivasa Rao, R.; Nagaiah, K. Silver phosphotungstate: A novel and recyclable heteropoly acid for Friedländer quinoline synthesis. Synthesis 2004, 2381–2385. [Google Scholar]
- Dabiri, M.; Baghbanzadeh, M.; Nikcheh, M.S. Oxalic acid: An efficient and cost-effective organic catalyst for the Friedlander quinoline synthesis under solvent-free conditions. Monatsh. Chem. 2007, 138, 1249–1252. [Google Scholar] [CrossRef]
- Shaabani, A.; Rahmati, A.; Badri, Z. Sulfonated cellulose and starch: New biodegradable and renewable solid acid catalysts for efficient synthesis of quinolines. Catal. Commun. 2008, 9, 13–16. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Arzroomchilar, E. 1-methylimidazolium triflouroacetate ([Hmim]TFA): An efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. Catal. Commun. 2008, 9, 939–942. [Google Scholar]
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Zolfigol, M.A.; Agheb, M.; Heydari, S. Silica sulfuric acid: An efficient reusable heterogeneous catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones in water and under solvent-free conditions. Catal. Commun. 2008, 9, 785–788. [Google Scholar] [CrossRef]
- Salehi, P.; Dabiri, M.; Zolfigol, M.A.; Otokesh, S.; Baghbanzadeh, M. Selective synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles in water at ambient temperature. Tetrahedron Lett. 2006, 47, 2557–2560. [Google Scholar] [CrossRef]
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Shakouri, M.; Otokesh, S.; Ekrami, T.; Doosti, R. Efficient and eco-friendly synthesis of dihydropyrimidinones, bis(indolyl) methanes, and N-alkyl and N-arylimides in ionic liquids. J. Iran. Chem. Soc. 2007, 4, 393–401. [Google Scholar] [CrossRef]
- Misono, M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem. Commun. 2001, 1141–1152. [Google Scholar] [CrossRef]
- Niknam, K; Zolfigol, M.A.; Dehghani, A. Friedländer quinoline synthesis catalyzed by M(HSO4)n (M=Al, Mg, Ca) under solvent-free conditions. Heterocycles 2008, 75, 2513–2521. [Google Scholar] [CrossRef]
- Waldmann, H.; Karunakar, G.V.; Kumar, K. Gold(III)-mediated aldol condensations provide efficient access to nitrogen heterocycles. Org. Lett. 2008, 10, 2159–2162. [Google Scholar] [CrossRef]
- Martinez, R.; Ramon, D.J.; Yus, M. RuCl2(DMSO)4 catalyzes the solvent-free indirect Friedländer synthesis of polysubstituted quinolines from alcohols. Eur. J. Org. Chem. 2007, 1599–1605. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dabiri, M.; Bashiribod, S. Phosphotungstic Acid: An Efficient, Cost-effective and Recyclable Catalyst for the Synthesis of Polysubstituted Quinolines. Molecules 2009, 14, 1126-1133. https://doi.org/10.3390/molecules14031126
Dabiri M, Bashiribod S. Phosphotungstic Acid: An Efficient, Cost-effective and Recyclable Catalyst for the Synthesis of Polysubstituted Quinolines. Molecules. 2009; 14(3):1126-1133. https://doi.org/10.3390/molecules14031126
Chicago/Turabian StyleDabiri, Minoo, and Sahareh Bashiribod. 2009. "Phosphotungstic Acid: An Efficient, Cost-effective and Recyclable Catalyst for the Synthesis of Polysubstituted Quinolines" Molecules 14, no. 3: 1126-1133. https://doi.org/10.3390/molecules14031126
APA StyleDabiri, M., & Bashiribod, S. (2009). Phosphotungstic Acid: An Efficient, Cost-effective and Recyclable Catalyst for the Synthesis of Polysubstituted Quinolines. Molecules, 14(3), 1126-1133. https://doi.org/10.3390/molecules14031126