Synthesis and Molecular Descriptor Characterization of Novel 4-Hydroxy-chromene-2-one Derivatives as Antimicrobial Agents
Abstract
:1. Introduction
2. Results and Discussion
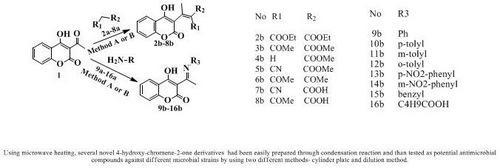
2.1. Synthesis
| No | R1 | R2 | Pw (W) | Tm(S) | Ta (ºC) | Yield (%) | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| 2b | COOEt | COOEt | 500 | 7 | 123 | 27 | 96 |
| 3b | COMe | COOMe | 500 | 7 | 129 | 36 | 97 |
| 4b | H | COOMe | 500 | 9 | 121 | 18 | 96 |
| 5b | C≡N | COOMe | 500 | 7 | 121 | 33 | 94 |
| 6b | COMe | COMe | 500 | 10 | 134 | 41 | 94 |
| 7b | C≡N | COOH | 500 | 6 | 120 | 42 | 84 |
| 8b | COMe | COOH | 500 | 5 | 126 | 35 | 87 |
| No | R3 | Pw (W) | Tm(min) | T (ºC) | Yield (%) | ||
| A | B | ||||||
| 9b | Ph | 500 | 3 | 109 | 75 | 95 | |
| 10b | p-tolyl | 500 | 3 | 109 | 73 | 97 | |
| 11b | m-tolyl | 500 | 3 | 109 | 84 | 94 | |
| 12b | o-tolyl | 500 | 3 | 109 | 73 | 94 | |
| 13b | p-NO2-phenyl | 500 | 3 | 109 | 51 | 92 | |
| 14b | m-NO2- phenyl | 500 | 3 | 109 | 62 | 97 | |
| 15b | benzyl | 500 | 3 | 109 | 75 | 97 | |
| 16b | C4H9COOH | 500 | 3 | 109 | 42 | 87 | |
| Parameters | Method | Compounds | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3b | 4b | 6b | 7b | 8b | 13b | ||
| Heat of Formation (kcal/mol) | PM3 | -120.57 | -184.38 | -149.91 | -140.02 | -119.39 | -193.17 | -20.97 |
| PM5 | -135.31 | -207.47 | -166.62 | -168.25 | -139.00 | -214.49 | -64.73 | |
| SPARTAN(e.u) | 26.97 | 57.58 | 57.26 | 70.91 | 40.23 | 41.66 | 72.38 | |
| Electronic Energy (eV) | PM3 | -14412.18 | -26955,66 | -21039,91 | -25237,76 | -22271,14 | -25448,75 | -27320,32 |
| Core-core Repulsion (EV) | PM3 | 11811.55 | 23083.21 | 17482.15 | 21395.99 | 18547.35 | 21441.45 | 23332.61 |
| Gradient Norm | PM3 | 414.37 | 430.48 | 447.56 | 383.06 | 115.66 | 69.75 | 320.41 |
| Dipole (debye) | PM3 | 7.32 | 4.99 | 7.25 | 6.11 | 6.54 | 4.96 | 9.94 |
| SPARTAN | 3.89 | 3.85 | 5.10 | 5.76 | 5.73 | 2.79 | 8.24 | |
| Symetry | PM3 | C1 | C1 | C1 | C1 | C1 | C1 | C1 |
| SPATRAN | C1 | C1 | C1 | C1 | C1 | C1 | C1 | |
| No. of Fields Level | PM3 | 38 | 57 | 49 | 54 | 50 | 54 | 60 |
| Ionization Potencial (eV) | PM3 | 9.70 | 9.41 | 9.34 | 9.34 | 9.67 | 9.50 | 9.59 |
| Homo Lumo Energies (eV) | PM3 | -9.70 | -9.41 | -9.34 | -9.34 | -9.67 | -9.50 | -9.60 |
| -1.26 | -1.30 | -1.37 | -1.30 | -1.59 | -1.31 | -1.45 | ||
| SPARTAN | -9.39 | -9.59 | -9.54 | -9.66 | -9.75 | -9.57 | -9.81 | |
| 1.26 | -1.29 | -1.26 | -1.26 | -1.50 | -1.32 | -1.55 | ||
| Molecular Weight | PM3 | 302.28 | 260.25 | 286.28 | 271.23 | 288.26 | 342.29 | |
| Scf Calculations | PM3 | 10 | 14 | 102 | 84 | 71 | 202 | 10 |





| Side chain atoms | Angle (º) | Dihedral atoms | Dihedral (º) |
|---|---|---|---|---|
| 1 | -C=C-CO | 120,67 | C (enol from ring)-C=C-CO | 0,21 |
| 3b | -C=C-CO | 122,43 | -C=C-C-O | -61,94 |
| -C=C-COO | 30,95 | -C=C-C-O (carbonyl) | -3,69 | |
| -C=C-C-C (ester) | -5,48 | |||
| 4b | -C=-C(from ring)-C(Me) | 115,93 | C=C(ring)-C (side chain)-C(Me) | -127,19 |
| -C=C-CO | 126,44 | -C=C-C-O (carbonyl) | -134,44 | |
| -C=C-C-C(ester) | 47,62 | |||
| 6b | C=C-C(carbonyl) | 121,79122,84 | C=C-C-O | 88,8615,34 |
| 7b | C=C-CN | 123,06 | C=C-C-C (cyano) | 3,28 |
| C=C-C(carboxyl acid) | 122,92 | -C=C-C-O (carbonyl) | -119,99 | |
| -C=C-C-O (ester) | 61,05 | |||
| 8b | -C=C-CO | 119,79 | -C=C-C-O (carbonyl) | -54,78 |
| C=C-C(carboxyl acid) | 124,68 | -C=C-C-O (carbonyl) | 154,01 | |
| -C=C-C-O(ester) | -30,51 | |||
| 13b | =C(from ring)-C=N- | 122,79 | =C (from ring)-C=N-C (aromatic) | 180 |
2.2. Minimal Inhibitory Concentration
| Cultures | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. aureus | M. lysodiekticus | E. coli | C. albicans | ||||||
| (isolate) | ||||||||||
| Comp. | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h |
| 1 | 0.09 | 0.19 | 0.94 | 1.90 | 0.19 | 0.38 | 0.19 | 0.38 | 0.09 | 0.09 |
| 3b | 0.13 | 0.50 | 0.50 | 1.00 | 0.13 | 0.50 | 0.13 | 0.50 | 0.13 | 0.50 |
| 4b | 0.13 | 0.50 | 0.50 | 1.00 | 0.13 | 0.50 | 0.13 | 0.50 | 0.25 | 0.50 |
| 6b | 0.13 | 0.25 | 0.13 | 0.50 | 0.50 | 0.50 | 0.25 | 1.00 | 0.13 | 0.25 |
| 7b | 0.13 | 1.00 | 0.50 | 0.50 | 0.13 | 0.50 | 0.50 | 1.00 | 0.50 | 1.00 |
| 8b | 0.13 | 0.50 | 0.50 | 1.00 | 0.13 | 0.50 | 0.13 | 0.50 | 0.13 | 0.13 |
| 13b | 0.13 | 0.50 | 0.13 | 0.50 | 0.50 | 0.50 | 0.13 | 0.50 | 0.13 | 0.13 |
2.3. Cylinder plate diffusion method
| Cultures a | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | |||||||||
| Time (h) | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| Comp. | Conc.(µg/mL) | Zones of inhibition (mm) b,c,d | |||||||||||||
| 1 | 75 | 15 | 16 | 10 | 10 | 8 | 8 | 6 | 7 | 17 | 17 | 10 | 12 | 26 | 30 |
| 10b | 75 | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| 150 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | |
| 13b | 75 | / | / | / | / | / | / | / | / | / | / | / | / | 22 | 23 |
| 150 | / | / | / | / | / | / | / | / | / | / | / | / | 30 | 37 | |
| 16b | 75 | 12 | 13 | 15 | 16 | / | / | 7 | 8 | 16 | 16 | 8 | 10 | 20 | 25 |
| Antibiotic | 10 | 26 | 24 | 20 | 19 | 24 | 25 | 52 | 53 | 24 | 23 | 21 | 21 | 26 | 23 |
3. Experimental
3.1. Chemistry

3.2. Molecular Descriptors
3.3. Microbiology
3.3.1. Culture of Microorganisms
3.3.2. Minimal Inhibitory Concentration
3.3.3. Disc diffusion Cylinder Plate Method
3.4. Instruments and apparatus
3.5. Preparation of 3-acetyl-4-hydroxy-chromene-2-one derivatives (general procedure)
3.5.1. Knoevenagel condensation of 3-acetyl-4-hydroxy-chromene-2-one (1) with carbonyl compounds 2a-8a
3.5.1.1. Method A - Conventional procedure
3.5.1.2. Method B - Microwave method
3.5.2. Condensation of 3-acetyl-4-hydroxy-chromene-2-one (1) with amines 9a-16a
3.5.2.1. Method A - Conventional method
3.5.2.2. Method B - Microwave method
3.6. Spectral data of synthesized coumarin derivatives
4. Conclusions
Acknowledgements
References
- Lovell, D.P.; Iersel, M.V.; Walters, D.; Price, R.J.; Lake, B.G. Genetic variation in the metabolism of coumarin in mouse liver. Pharmacogenetics 1999, 9, 239–250. [Google Scholar]
- Gregory, J.F.; Emma, K.; Bernadette, S.C.; Denise, A.E. In vitro cytotoxic potential and mechanism of action of selected coumarins, using human renal cell lines. Cancer Lett. 2002, 183, 61–68. [Google Scholar]
- Kirkiacharian, S.; Thuy, T.; Sicsic, S.; Bakhchinian, R.; Kurkjian, R.; Tonnaire, T. Structure-activity relationships of some 3-substituted-4-hydroxycooumarins as HIV-1 protease inhibitors. Farmaco 2002, 57, 703–708. [Google Scholar] [CrossRef]
- Lafitte, D.; Lamour, V.; Tsvetkov, P.; Markov, A.A.; Deprez, M.; Klich, P.; Moras, D.; Briand, C.; Gilli, R. DNA gyrase intgeraction with coumarin-based inhibitors-the role of the hydroxybenzoate isopentenyl moiety and the 5,-methyl group of the noviose. Biochemistry 2002, 41, 7217–7223. [Google Scholar] [CrossRef]
- Hurry, R.G.; Cortz, C.; Ananthanaraxan, T.P.; Schmolka, S. A new coumarin synthesis and its utilization for the synthesis of polycyclic coumarin compounds with anticarcinogenic properties. J. Org. Chem. 1998, 53, 3936–3943. [Google Scholar]
- Traykova, M.; Kostova, I. Coumarin Derivatives and Oxidative Stress. Int. J. Pharm. 2005, 1, 29–32. [Google Scholar] [CrossRef]
- O`Kennedy, R.; Thornes, R.D.; Richard, O.K.; Douglas, T.R. Coumarins: Biology, Applications and Mode of Action; John Wiley and Sons: Chichester, UK, 1977. [Google Scholar]
- Zahradnik, M. The Producton and Application of Fluorescent Brightening Agents; John Wiley and Sons: New York, NY, USA, 1992. [Google Scholar]
- Maeda, M. Laser Dyes; Academic Press: New York, USA, 1994. [Google Scholar]
- Kostova, I. Syntetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Westaway, H.; Ali, H.; Baldivera, L.; Laberge, L.; Rousell. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279. [Google Scholar] [CrossRef]
- Ambramovitch, R.A. Applications of microwave energy in organic chemistry. A review. Org. Prep. Proc. Int. 1991, 23, 683. [Google Scholar] [CrossRef]
- Bose, A.K.; Manhas, M.S.; Ghost, M.; Raju, V.S.; Tabei, K.; Urbanczyk-Lipowska, Z. Examination of the Perkin Reaction under Microwave Irradiation. Heterocycles 1990, 30, 741. [Google Scholar] [CrossRef]
- Villemin, D.; Ben Alloum, A. Potassium fluoride on alumina: condensation of 3-methyl-2-thiono-4-thiazolidinone with aldehydes. Synthesis of α-thioacrylic acids phosphonothionothiazolidinones. Phosph. Sulf. Silic. 1993, 79, 33. [Google Scholar] [CrossRef]
- Puciová, M.; Ertl, P.; Toma, S. Synthesis of ferrocenyl-substituted heterocycles: the beneficial effect of the microwave irradiation. Collect. Czech Chem. Commun. 1994, 59, 175. [Google Scholar] [CrossRef]
- Lácová, M.; Chovancová, J.; Veverková, E.; Toma, Š. Microwaves assisted Gabriel synthesis of phthalides. Tetrahedron 1996, 52, 14995–15006. [Google Scholar] [CrossRef]
- Hamelin, J.; Bazureau, J.P.; Texier-Boullet, F.; Loupy, A. Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, 2002; p. 253. [Google Scholar]
- Besson, T.; Brain, C.T.; Lidström, P.; Tierney, J.P. Microwave-Assisted Organic Synthesis; Blackwell Publishing: Oxford, 2004; p. 44. [Google Scholar]
- Kappe, C.O.; Stadtler, A. Microwaves in Organic and Medicinal Chemistry; Wiley-CH: Weinheim, 2005. [Google Scholar]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Milosevic, T. Synthesis and antimicrobial evaluation of some novel 2-aminothiazole derivatives of 4-hydroxy-chromene-2-one. Arch. Pharm. 2008, 341, 491–496. [Google Scholar] [CrossRef]
- Sukdolak, S.; Solujić, S.; Vuković, N.; Manojlović, N.; Krstić, Lj. Synthesis of 3-(thiazol-4-yl)-4-hydroxycoumarins. J. Serb. Chem. Soc. 2004, 69, 319–326. [Google Scholar] [CrossRef]
- Sukdolak, S.; Solujić, S.; Manojlović, N.; Vuković, N.; Krstić, Lj. Hantzsch reaction of 3-(2-bromoacetyl)-4-hydroxy-chromen-one. J. Heterocycl. Chem. 2004, 41, 593–596. [Google Scholar] [CrossRef]
- Sukdolak, S.; Vuković, N.; Solujić, S.; Milošev, M.; Manojlović, N.; Krstić, Lj. Synthesis of new 3-(2-aminothiazol-4-yl)-4-hydroxy-chromen-2-one derivatives. J. Serb. Chem. Soc. 2006, 71, 6. [Google Scholar]
- Vuković, N.; Sukdolak, S.; Solujić, S.; Manojlović, N.; Krstić, Lj. Synthesis of some 3-(2-aminothiazol-4-yl)-4-hydroxycoumarins. In Proceedings of the XVIII Congress of Chemists and Technologists of Macedonia, Ohrid, 2004. OCB-15.
- Vuković, N.; Sukdolak, S.; Solujić, S.; Manojlović, N.; Krstić, L. Synthesis of 3-(thiazol-4-yl)-4-hydroxy Coumarins. In Book of Abstracts of the 4th International Conference of the Chemical Societes of the Sout-East Europian Countries, Belgrade, 2004. GT-P 139.
- Bram, G.; Loupy, A.; Villemin, D. Solid Supports and Catalysts in Organic Chemistry; Ellis Harwood: London, UK, 1992. [Google Scholar]
- Software Mopac 2000. version 11. ChemOffice Ultra, 2006. Cambridge software, 2006.
- Software CAChe WorkSystem Pro 6.01. Textronix WorkSystem, 2003.
- Software Spartan 2002 for Windows. Wavefunction, Inc.: USA, 2002.
- Kayser, O.; Koldrziey, H. Antibacterial activity of simple coumarins structural requirements for biological activity. Z. Naturforch. 1999, 54c, 169–174. [Google Scholar]
- Rauchman, B.S.; Tidwell, M.X.; Johnson, J.V.; Roth, B. 2,4,diamino-5-benzyl-pirimidines and analogues as antibacterial agents. J. Med, Chem. 1989, 32, 1927–1935. [Google Scholar] [CrossRef]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharm. 2006, 73, 299–305. [Google Scholar]
- Montagner, C.; de Souza, S.; Groposoa, C.; Delle Monache, F.; Smania, E.; Smania, A. Antifungal activity of coumarins. J. Biosci. 2008, 63, 21–28. [Google Scholar]
- Elinos-Báez, C.M.; Leon, F.; Sants, E. Effects of coumarin and 7OH-coumarin on bcl-2 and Bax expression in two human lung cancer cell lines in vitro. Cell Biol. Int. 2005, 29, 703–708. [Google Scholar] [CrossRef]
- Duncana, S.H.; Flinta, H.J.; Stewarta, C.S. Inhibitory activity of gut bacteria against Escherichia coli O157 mediated by dietary plant metabolites. FEMS Microbiol. Letters 2006, 164, 283–288. [Google Scholar]
- Teles, H.L.; Sordi, R.; Silva, G.H.; Castro-Gamboa, I.; da Silva Bolzani, V.; Pfenning, L.H.; Magalhães de Abreu, L.; Costa-Neto, C.M.; Young, M.C.M.; Araújo, Â.R. Aromatic compounds produced by Periconia atropurpurea, an endophytic fungus associated with Xylopia aromatic. Phytochemistry 2006, 67, 2686–2690. [Google Scholar] [CrossRef]
- NCCLS. Performance standards for antimicrobial susceptibility testing 14th Int. Suplement M100-S14; Wayne: PA, USA, 2003. [Google Scholar]
- Umesha, S.; Richardson, P.A.; Kong, P; Hong, C.X. A novel indicator plant to test the hypersensitivity of phytopathogenic bacteria. J. Microbiol. Meth. 2008, 72, 95–97. [Google Scholar] [CrossRef]
- Carter, C.H.; Gaspar, A.J.; Leise, J.M. Resazurin staining of bacterial colonies on membranes filters.; Fort Dietrick: Frederick, Maryland, USA, 1955. [Google Scholar]
- Beena, J.; Akhila, R.; Emilia, A. Antimicrobial activity and chemical composition of essential oil from Hedychium coronarium. Phytother. Res. 2007, 21, 439–443. [Google Scholar] [CrossRef]
- Massada, Y. Analysis of essential oil by gas chromatography and spectrometry; Wiley: New York, USA, 1976. [Google Scholar]
- BSAC. Disc Diffusion Method for Antimicrobial Susceptibility Testing, Verision 4; 2005. [Google Scholar]
- CLSI. Performance Standards for antimicrobial Disc Susceptibility Tests Approved Standard- Tenth Edition, M02-A10; Wayne, PA, 2009. [Google Scholar]
- Sample Availability: Samples of the compounds 2b-15b are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mladenović, M.; Vuković, N.; Nićiforović, N.; Sukdolak, S.; Solujić, S. Synthesis and Molecular Descriptor Characterization of Novel 4-Hydroxy-chromene-2-one Derivatives as Antimicrobial Agents. Molecules 2009, 14, 1495-1512. https://doi.org/10.3390/molecules14041495
Mladenović M, Vuković N, Nićiforović N, Sukdolak S, Solujić S. Synthesis and Molecular Descriptor Characterization of Novel 4-Hydroxy-chromene-2-one Derivatives as Antimicrobial Agents. Molecules. 2009; 14(4):1495-1512. https://doi.org/10.3390/molecules14041495
Chicago/Turabian StyleMladenović, Milan, Nenad Vuković, Neda Nićiforović, Slobodan Sukdolak, and Slavica Solujić. 2009. "Synthesis and Molecular Descriptor Characterization of Novel 4-Hydroxy-chromene-2-one Derivatives as Antimicrobial Agents" Molecules 14, no. 4: 1495-1512. https://doi.org/10.3390/molecules14041495
APA StyleMladenović, M., Vuković, N., Nićiforović, N., Sukdolak, S., & Solujić, S. (2009). Synthesis and Molecular Descriptor Characterization of Novel 4-Hydroxy-chromene-2-one Derivatives as Antimicrobial Agents. Molecules, 14(4), 1495-1512. https://doi.org/10.3390/molecules14041495