A New Sesquiterpene from the Fruits of Daucus carota L.
Abstract
:Introduction
Results and Discussion
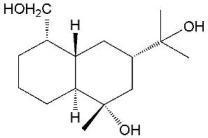
Structure Elucidation


Cytotoxicity Assay
Experimental
General
Plant Material
Extraction and Isolation
Cytotoxicity Assay
Conclusions
Supplementary Files
Supplementary File 1References and Notes
- Pant, B.; Manandhar, S. In vitro propagation of carrot (Daucus carota). Scientific World 2007, 5, 51. [Google Scholar]
- Rossi, P.G.; Bao, L.; Luciani, A.; Panighi, J.; Desjobert, J.M.; Costa, J.; Casanova, J.; Bolla, J. M.; Berti, L. (E)-methylisoeugenol and elemicin: antibacterial components of Daucus carota L. essential oil against Campylobacter jejuni. J. Agr. Food Chem. 2007, 55, 7332–7336. [Google Scholar] [CrossRef]
- Tavares, A.C.; Goncalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Lopes, M.C.; Canhoto, J.; Salgueiro, L.R. Essential oil of Daucus carota subsp. halophilus: Composition, antifungal activity and cytotoxicity. J. Ethnopharmacol. 2008, 119, 129–134. [Google Scholar] [CrossRef]
- Bishayee, A.; Sarkar, A.; Chatterjee, M. Hepatoprotective activity of carrot (Daucus carota L.) against carbon tetrachloride intoxication in mouse liver. J. Ethanopharmacol. 1995, 47, 69–74. [Google Scholar] [CrossRef]
- Yang, R.L.; Yan, Z.H.; Lu, Y. Cytotoxic Phenylpropanoids from Carrot. J. Agr. Food Chem. 2008, 56, 3024–3027. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Bishr, M.M.; El-Shanawany, M.A.; Attia, E.Z.; Ross, S.A.; Pare, P.W. Rare trisubstituted sesquiterpenes daucanes from the wild Daucus carota. Phytochemistry 2005, 66, 1680–1684. [Google Scholar] [CrossRef]
- Dhillon, R.S.; Gautam, V.K.; Kalsi, P.S.; Chhabra, B.R. Carota-1,4-β-oxide, a sesquiterpene from Daucus carota. Phytochemistry 1989, 28, 639–640. [Google Scholar] [CrossRef]
- Cool, L.G. Ent-Daucane and acorane sesquiterpenes from Cupressocyparis leylandii foliage. Phytochemistry 2001, 58, 969–972. [Google Scholar] [CrossRef]
- Czepa, A.; Hofmann, T. Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree. J. Agr. Food Chem. 2003, 51, 3865–3873. [Google Scholar] [CrossRef]
- Gebhardt, Y.; Witte, S.; Forkmann, G.; Lukacin, R.; Matern, U.; Martens, S. Molecular evolution of flavonoid dioxygenases in the family Apiaceae. Phytochemistry 2005, 66, 1273–1284. [Google Scholar] [CrossRef]
- Gupta, K.R.; Niranjan, G.S. A new flavone glycoside from seeds of Daucus carota. Planta Med. 1982, 46, 240–241. [Google Scholar] [CrossRef]
- Ivie, G.W.; Beier, R.C.; Holt, D.L. Analysis of the garden carrot (Daucus carota L.) for linear furocoumarins (psoralens) at the sub parts per million level. J. Agr. Food Chem. 1982, 30, 413–416. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Clevidence, B.A.; Britz, S.J.; Simon, P.W.; Novotny, J.A. Plasma and urine responses are lower for acylated vs nonacylated anthocyanins from raw and cooked purple carrots. J. Agr. Food Chem. 2005, 53, 6537–6542. [Google Scholar] [CrossRef]
- Hemingson, J.C.; Collins, R.P. Anthocyanins present in cell cultures of Daucus carota. J. Nat. Prod. 1982, 45, 385–389. [Google Scholar] [CrossRef]
- Fu, H.W.; Zhang, H.L.; Pei, Y.H. A new coumestan from Arachis hypogaea L. Chinese Chem. Lett. 2005, 16, 918–920. [Google Scholar]
- Li, F.; Li, W.; Fu, H.W.; Zhang, Q.B.; Koike, K. Pancreatic lipase-inhibiting triterpenoid saponins from fruits of Acanthopanax senticosus. Chem. Pharm. Bull. 2007, 55, 1087–1089. [Google Scholar] [CrossRef]
- Wang, N.; Li, Z.L.; Song, D.D.; Li, W.; Fu, H.W.; Koike, K.; Pei, Y.H.; Jing, Y.K.; Hua, H.M. Lanostane-type triterpenoids from the roots of Kadsura coccinea. J. Nat. Prod. 2008, 71, 990–994. [Google Scholar] [CrossRef]
- Chang, X.L.; Li, W.; Jia, Z.H.; Satou, T.; Fushiya, S.J.; Koike, K. Biologically active triterpenoid saponins from Ardisia japonica. J. Nat. Prod. 2007, 70, 179–187. [Google Scholar] [CrossRef]
- Li, Y.L.; Gan, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huang, Y.P.; Liu, Y.W.; Liu, J.W. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound 1 for research is available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fu, H.-W.; Zhang, L.; Yi, T.; Tian, J.-K. A New Sesquiterpene from the Fruits of Daucus carota L. Molecules 2009, 14, 2862-2867. https://doi.org/10.3390/molecules14082862
Fu H-W, Zhang L, Yi T, Tian J-K. A New Sesquiterpene from the Fruits of Daucus carota L. Molecules. 2009; 14(8):2862-2867. https://doi.org/10.3390/molecules14082862
Chicago/Turabian StyleFu, Hong-Wei, Lin Zhang, Tao Yi, and Jing-Kui Tian. 2009. "A New Sesquiterpene from the Fruits of Daucus carota L." Molecules 14, no. 8: 2862-2867. https://doi.org/10.3390/molecules14082862
APA StyleFu, H. -W., Zhang, L., Yi, T., & Tian, J. -K. (2009). A New Sesquiterpene from the Fruits of Daucus carota L. Molecules, 14(8), 2862-2867. https://doi.org/10.3390/molecules14082862