Synthesis and Vasorelaxant and Platelet Antiaggregatory Activities of a New Series of 6-Halo-3-phenylcoumarins †
Abstract
:1. Introduction
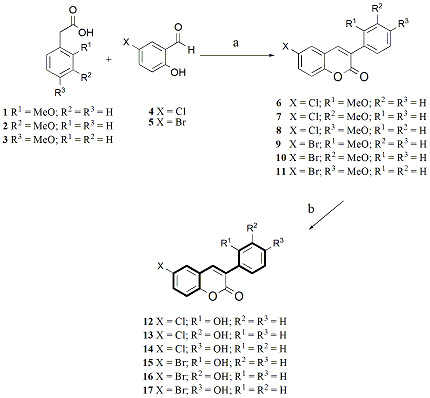
2. Results and Discussion
3. Experimental
3.1. General
3.2. Chemistry
3.3. Vasorelaxant activity
3.4. Antiplatelet activity
4. Conclusions
Acknowledgements
References and Notes
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, V.B.; Scholtholt, J. Effects of carbocromene on myocardial oxygen consumption in isolated dog hearts. J. Pharmacol. Exp. Ther. 1981, 217, 306–313. [Google Scholar] [PubMed]
- Opherk, D.; Schuler, G.; Waas, W.; Dietz, R.; Kubler, W. Intravenous carbochromen: A potent and effective drug for estimation of coronary dilatory capacity. Eur. Heart J. 1990, 11, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.G.; Liu, X.Q.; Chen, Y.C.; Hao, K.; Wang, G.J. Warfarin maintenance dose adjustment with indirect pharmacodynamic model in rats. Eur. J. Pharm. Scien. 2007, 30, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F.; Alvarez, E.; Camina, M.; Leiro, J.M.; Gomez, E.; Fernandez, P. The possible implication of trans-Resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol. Pharmacol. 2002, 61, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Wang, Z.R.; Hsieh, T.C.; Bruder, J.L.; Zou, J.G.; Huang, Y.Z. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine. Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.D.; He, L.R. J. Mechanisms of cardiovascular protection by resveratrol. Med. Food 2004, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Quezada, E.; Santana, L.; Uriarte, E.; Yanez, M.; Fraiz, N.; Alcaide, C.; Cano, E.; Orallo, F. Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids. Bioorg. Med. Chem. Lett. 2006, 16, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Oda, N.; Yoshida, Y.; Nagai, S.; Ueda, T.; Sakakibara, J. Synthesis of coumarins by Nucleophilic Denitrocyclization Reaction. Chem. Pharm. Bull. 1987, 35, 1796–1802. [Google Scholar] [CrossRef]
- Hans, N.; Singhi, M.; Sharma, V.; Grover, S.K. A novel one-step synthesis of 3-phenyl-, 4-methyl-3-phenyl- and 3-phenyl-4-styrylcoumarins using DCC-DMSO. Indian J. Chem. 1996, 35B, 1159–1162. [Google Scholar] [CrossRef]
- Perkin, W.H. On the artificial production of coumarin and formation of its homologues. J. Chem. Soc. 1868, 21, 53–63. [Google Scholar] [CrossRef]
- Perkin, W.H. On the formation of coumarin and of cinnamic and of other analogous acids from the aromatic aldehydes. 1877, 31, 388–427. [Google Scholar] [CrossRef]
- Begala, M.; Delogu, G.; Maccioni, E.; Podda, G.; Tocco, G.; Quezada, E.; Uriarte, E.; Fedrigo, M.A.; Favretto, D.; Traldi, P. Electrospray ionisation tandem mass spectrometry in the characterisation of isomeric benzofurocoumarin. Rapid Commun. Mass Spectrom. 2001, 15, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.; Zimmer, H.; Purcell, T.C. Synthesis and cyclization reactions of 3-(2-hydroxybenzylidene)-2(3H)-coumaranones. J. Org. Chem. 1966, 31, 3854–3857. [Google Scholar] [CrossRef] [PubMed]
- Mimai, M.; Suzuki, Y.; Hamma, N.; Murayama, E.; Aono, S. Coumarin derivatives. JP Pat. 49054371, 1974. [Google Scholar]
Sample Availability: Contact the authors. |


| Compound | PE (1 μM) |
|---|---|
| 12 | 36.63 ± 2.46* |
| 13 | 57.63 ± 3.87* |
| 14 | ** |
| 15 | 48.79 ± 3.27* |
| 16 | 46.67 ± 3.13* |
| 17 | ** |
| t-RESV | 3.12 ± 0.26 |
| Compound | Thrombin (0.25 U/mL) |
|---|---|
| 12 | 91.36 ± 6.13* |
| 13 | 6.41 ± 2.15* |
| 14 | ** |
| 15 | ** |
| 16 | ** |
| 17 | 20.1 ± 1.35* |
| t-RESV | 195.50 ± 13.82 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Quezada, E.; Delogu, G.; Picciau, C.; Santana, L.; Podda, G.; Borges, F.; García-Morales, V.; Viña, D.; Orallo, F. Synthesis and Vasorelaxant and Platelet Antiaggregatory Activities of a New Series of 6-Halo-3-phenylcoumarins. Molecules 2010, 15, 270-279. https://doi.org/10.3390/molecules15010270
Quezada E, Delogu G, Picciau C, Santana L, Podda G, Borges F, García-Morales V, Viña D, Orallo F. Synthesis and Vasorelaxant and Platelet Antiaggregatory Activities of a New Series of 6-Halo-3-phenylcoumarins. Molecules. 2010; 15(1):270-279. https://doi.org/10.3390/molecules15010270
Chicago/Turabian StyleQuezada, Elías, Giovanna Delogu, Carmen Picciau, Lourdes Santana, Gianni Podda, Fernanda Borges, Verónica García-Morales, Dolores Viña, and Francisco Orallo. 2010. "Synthesis and Vasorelaxant and Platelet Antiaggregatory Activities of a New Series of 6-Halo-3-phenylcoumarins" Molecules 15, no. 1: 270-279. https://doi.org/10.3390/molecules15010270
APA StyleQuezada, E., Delogu, G., Picciau, C., Santana, L., Podda, G., Borges, F., García-Morales, V., Viña, D., & Orallo, F. (2010). Synthesis and Vasorelaxant and Platelet Antiaggregatory Activities of a New Series of 6-Halo-3-phenylcoumarins. Molecules, 15(1), 270-279. https://doi.org/10.3390/molecules15010270