An Expeditious Synthesis of N-substituted Pyrroles via Microwave-Induced Iodine-Catalyzed Reactions under Solventless Conditions
Abstract
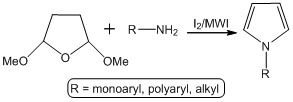
:1. Introduction

2. Results and Discussion

 |
3. Experimental
3.1. General
3.2. General procedure for the synthesis of pyrroles (3)
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Lainton, J.A.H.; Hoffman, J.W.; Martin, D.R.; Compton, D.R. 1-Alkyl-3-(1-naphthoyl)pyrroles: a new class of cannabinoid. Tetrahedron Lett. 1995, 36, 1401–1404. [Google Scholar] [CrossRef]
- de Leon, C.Y.; Ganem, B. A new approach to porphobilinogen and its analogs. Tetrahedron 1997, 53, 7731–7752. [Google Scholar] [CrossRef]
- Gilchrist, T.L. Synthesis of aromatic heterocycles. J. Chem. Soc., Perkin Trans 1 1998, 615–628, and references cited therein. [Google Scholar] [CrossRef]
- Ruault, P.; Pilard, J.-F.; Touaux, B.; Boullet, F.T.; Hamelin, J. Rapid generation of amines by microwave irradiation of ureas dispersed on clay. Synlett 1994, 935–936. [Google Scholar]
- Danks, T.N. Microwave assisted synthesis of pyrroles. Tetrahedron Lett. 1999, 40, 3957–3960. [Google Scholar] [CrossRef]
- Becker, F.F.; Banik, B.K. Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of some chrysene derivatives. Bioorg. Med. Chem. Lett. 1998, 8, 2877–2880. [Google Scholar] [CrossRef]
- Becker, F.F.; Mukhopadhyay, C.; Hackfeld, L.; Banik, I.; Banik, B.K. Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of dibenzofluorene derivatives. Bioorg. Med. Chem. 2000, 8, 2693–2699. [Google Scholar] [CrossRef]
- Banik, B.K.; Becker, F.F. Polycyclic aromatic compounds as anticancer agents. 4. Structure-activity relationships of chrysene and pyrene derivatives. Bioorg. Med. Chem. 2001, 9, 593–605. [Google Scholar] [CrossRef]
- Banik, B.K.; Becker, F.F. Synthesis, electrophilic substitution and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents. Curr. Med. Chem. 2001, 8, 1513–1533. [Google Scholar] [CrossRef]
- Banik, I.; Becker, F.F.; Banik, B.K. Stereoselective Synthesis of β-Lactams with Polyaromatic Imines: Entry to New and Novel Anticancer Agents. J. Med. Chem. 2003, 46, 12–15. [Google Scholar] [CrossRef]
- Banik, B.K.; Manhas, M.S.; Bose, A.K. StereospecificGlycosylation via Ferrier Rearrangement for Optical Resolution. J. Org. Chem. 1994, 59, 4714–4716. [Google Scholar] [CrossRef]
- Banik, B.K.; Manhas, M.S.; Bose, A.K. Studies on lactams. 103. Enantiopure α-hydroxy-β-lactams via stereoselective glycosylation. Tetrahedron Lett. 1997, 38, 5077–5080. [Google Scholar] [CrossRef]
- Banik, B.K.; Zegrocka, O.; Manhas, M.S.; Bose, A.K. Studies on lactams. 104. Enantiomerically pure β-lactams with the thienamycin side chain via glycosylation. Heterocycles 1997, 27, 173–176. [Google Scholar]
- Banik, B.K.; Fernandez, M.; Alvarez, C. Iodine-catalyzed highly efficient Michael reaction of indoles under solvent-free condition. Tetrahedron Lett. 2005, 46, 2479–2482. [Google Scholar]
- Banik, B.K.; Garcia, I.; Morales, F.; Aguilar, C. Novel synthesis of substituted pyrrole bound to indolinone via molecular iodine-catalyzed reaction. Heterocycl. Commun. 2007, 13, 109–112. [Google Scholar]
- Banik, B.K.; Garza, R. Molecular iodine-catalyzed deprotection of acetals and ketals in acetone. Chem. Edu. 2007, 12, 75–76. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bandyopadhyay, D.; Mukherjee, S.; Banik, B.K. An Expeditious Synthesis of N-substituted Pyrroles via Microwave-Induced Iodine-Catalyzed Reactions under Solventless Conditions. Molecules 2010, 15, 2520-2525. https://doi.org/10.3390/molecules15042520
Bandyopadhyay D, Mukherjee S, Banik BK. An Expeditious Synthesis of N-substituted Pyrroles via Microwave-Induced Iodine-Catalyzed Reactions under Solventless Conditions. Molecules. 2010; 15(4):2520-2525. https://doi.org/10.3390/molecules15042520
Chicago/Turabian StyleBandyopadhyay, Debasish, Sanghamitra Mukherjee, and Bimal K. Banik. 2010. "An Expeditious Synthesis of N-substituted Pyrroles via Microwave-Induced Iodine-Catalyzed Reactions under Solventless Conditions" Molecules 15, no. 4: 2520-2525. https://doi.org/10.3390/molecules15042520
APA StyleBandyopadhyay, D., Mukherjee, S., & Banik, B. K. (2010). An Expeditious Synthesis of N-substituted Pyrroles via Microwave-Induced Iodine-Catalyzed Reactions under Solventless Conditions. Molecules, 15(4), 2520-2525. https://doi.org/10.3390/molecules15042520