Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer
Abstract
:1. Introduction
2. Classification of Plant Phenolics

3. Phenolics from A. annua


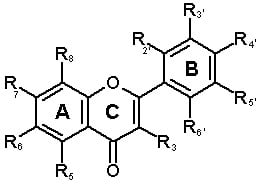
| Structure | Phenolic type | Ring and substituent position | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Flavones | C(R3) | A(R5) | A(R6) | A(R7) | A(R8) | B(R2’) | B(R3’) | B(R4’) | B(R5’) | B(R6’) |
| 7 | Apigenin | H | OH | H | OH | H | H | H | OH | H | H |
| 8 | Luteolin (5,7,3’,4’-Tetrahydroxy flavone) | H | OH | H | OH | H | H | OH | OH | H | H |
| 9 | Luteolin-7-methylether | H | OH | H | OCH3 | H | H | OH | OH | H | H |
| 10 | Acacetin (apigenin-4’-methyl ether) or 5,7-dihydroxy-4-methoxy flavone | H | OH | H | OH | H | H | H | OCH3 | H | H |
| 11 | Chrysoeriol (Lutoelin-3’-methyl ether) or5,7,4’-Trihydroxy-3’-methoxy flavone | H | OH | H | OH | H | H | OCH3 | OH | H | H |
| 12 | Chrysin (5,7-Dihydroxy flavone) | H | OH | H | OH | H | H | H | H | H | H |
| 13 | Cirsilineol (6-Hydroxyluteolin-6,7,3’-trimethyl ether or 5,4’-dihydroxy-6,7,3’-trimethoxyflavone, Fastigenin, Anisomelin, Eupatrin) | H | OH | OCH3 | OCH3 | H | OH | OCH3 | OH | H | H |
| 15 | Cynaroside (Luteolin-7-glucoside or 5,7,3’,4’-Tetrahydroxyflavone-7-glucoside or Glucoluteolin or Luteoloside or Cinaroside) | H | OH | H | OGlu | H | H | OH | OH | H | H |
| 16 | Eupatorin (6-Hydroxyluteolin-6,7,4’-trimethyl ether or 5,3’-Dihydroxy-6,7,4’-trimethoxyflavone) | H | OH | OCH3 | OCH3 | H | H | OH | OCH3 | H | H |
| 17 | Cirsimaritin (Scutellarin-6,7-dimethyl ether or 6-Hydroxyapigenin-6,7-dimethyl ether or 5,4’-Dihydroxy-6,7-Dimethoxyflavone or Scorphulein or Cirsumaritin or Cirsitakaogenin) | H | OH | OCH3 | OCH3 | H | H | H | OH | H | H |
| 18 | Artemetin | OCH3 | OH | OCH3 | OCH3 | H | H | OCH3 | OCH3 | H | H |
| 19 | Chrysosplenol-C | OCH3 | OH | OH | OCH3 | H | H | OCH3 | OH | H | H |
| 20 | Chrysosplenol-D | OCH3 | OH | OCH3 | OCH3 | H | H | OH | OH | H | H |
| 21 | Mikanin | OH | OH | OCH3 | OCH3 | H | H | H | OCH3 | H | H |
| 22 | Astragalin (Kaempferol-3-α-D-glucoside) | O-glu | OH | H | OH | H | H | H | OH | H | H |
| 23 | Axillarin (5,7,3’,4’-Tetrahydroxy-3,6-dimethoxyflavone or quercetagetin -3,6- dimethyl ether) | OCH3 | OH | OCH3 | OH | H | H | OH | OH | H | H |
| 24 | Casticin (5,3’-dihydroxy-3,6,7,4’-tetramethyl ether flavone or Quercetagetin -3,6-7,4’-tetramethyl ether) | OCH3 | OH | OCH3 | OCH3 | H | H | OH | OCH3 | H | H |
| 25 | Eupatin (3,5,3’-Trihydroxy-6,7,4’-trimethoxyflavone or Quercetagetin -3,6- dimethyl ether) | OH | OH | OCH3 | OCH3 | H | H | OH | OCH3 | H | H |
| 26 | Kaempferol (3,5,7,4’-Tetrahydroxy flavone) | OH | OH | H | OH | H | H | H | OH | H | H |
| 27 | Kaempferol-6-methox-3-O-β-D-glucoside | OGlu | OH | OCH3 | OH | H | H | H | OH | H | H |
| 28 | Tamarixetin | OH | OH | H | OH | H | H | OH | OCH3 | H | H |
| 29 | Myricetin (3,5,7,3’,4’,5’-Hexahydroxy flavone) | OH | OH | H | OH | H | H | OH | OH | OH | H |
| 30 | Gossypetin- 3,8-dimethylether | OCH3 | OH | H | OH | OH | H | OH | OCH3 | H | H |
| 31 | Laricitrin (3,5,7,3’,4’, -Pentahydroxy 5’-methoxyflavone) | OH | OH | H | OH | H | H | OH | OH | OCH3 | H |
| 32 | Mearnsetin (3,5,7,3’,5’, -Pentahydroxy 4’-methoxyflavone or Myricetin-4-methyl ether) | OH | OH | H | OH | H | H | OH | OCH3 | OH | H |
| 33 | Quercetin (3,5,7,3’,4’-Pentahydroxy flavone) | OH | OH | H | OH | H | H | OH | OH | H | H |
| 34 | Quercetin-3’- O-β-D-glucoside | OH | OH | H | OH | H | H | O-Glu | OH | H | H |
| 35 | Quercetin-3- methylether | OCH3 | OH | H | OH | H | H | OH | OH | H | H |
| 36 | Quercimeritrin (Quercetin-7-glucoside) | OH | OH | H | O-Glu | H | H | OH | OH | H | H |
| 37 | Retusin (5-Hydroxy-3,7,3’4’-tetramethoxy flavone or Quercetin3,7,3’,4’-tetramethylether) | OCH3 | OH | H | OCH3 | H | H | OCH3 | OCH3 | H | H |
| 38 | Rhamnetin (Quercetin-7-methylether or 3,5,7,3’-Tetrahydroxy-4’-methoxy flavone) | OH | OH | H | OCH3 | H | H | OH | OH | H | H |
| 39 | Isorhamnetin (Quercetin-3’-methylether or 3,5,7,4’-Tetrahydroxy-3’-methoxy flavone) | OH | OH | H | OH | H | H | OCH3 | OH | H | H |
| 40 | Rutin (Quercetin-3-rutinoside) | O-Diglyc. | OH | H | OH | H | H | OH | OH | H | H |
| 41 | Mearncetin glucoside | OH | OH | H | OGlu | H | H | OH | OCH3 | OH | H |
| 42 | Chrysosplenetin (5,4’-Dihydroxy-3,6,7,3’-tetramethoxy flavone or Quercetagetin-3,6-7,3’-tetramethyl ether) | OCH3 | OH | OCH3 | OCH3 | H | H | OCH3 | OH | H | H |
| 43 | 3,5-Dihydroxy-3’,4’,6,7,-Tetramethoxyflavone | OH | OH | OCH3 | OCH3 | H | H | OCH3 | OCH3 | H | H |
| 44 | Syringetin (Myricetin-3’,5’-dimethyl ether) | OH | OH | H | OH | H | H | OCH3 | OH | OCH3 | H |
| 45 | Isokaempferide (5,7,4’-Trihydroxy-3-methoxyflavone or Kaemferol-3-methyl ether) | OCH3 | OH | H | OH | H | H | H | OH | H | H |
| 46 | Quercetagetin 3,4’-dimethyl ether | OCH3 | OH | OH | OH | H | H | OH | OCH3 | H | H |
4. A. annua Has Different Chemotypes
5. Antioxidant vs. Biological Activity of Flavonoids
6. Antioxidant Activity of A. annua Flavonoids
7. Antimalarial Activity of Flavonoids
8. Antimalarial Activity of A. annua Flavonoids
9. Flavonoids and Cancer
10. Anticancer Activity of Artemisinin and A. annua Flavonoids
| Flavonoids | Mean GI50 (μM) [74] | Synergy with anti-cancer agents |
|---|---|---|
| Eupatin | 4 | mitoxantrone [75] |
| Silybin | Paclitaxel [76], TRAIL [77], SN-38 [78], mitoxantrone [78], cisplatin [79], carboplatin [79] | |
| Quercetin | 60 | TRAIL [80], cisplatin [81], doxorubicin [82], vinblastine [83], paclitaxel [83], gemcitabine [84], topotecan [84] |
| Apigenin | 27a | TRAIL [85,86], tamoxifen [87], fulvestrant [87] |
| Luteolin | N/A | Rapamycin [88], doxorubicin [89], cisplatin [90], TRAIL [91] |
| Kaempferol | N/A | TRAIL [92,93], vinblastine [83], paclitaxel [83], mitoxantrone [78] |
10.1. Flavones
10. 2. Flavonols
11. Flavonoid Metabolism
12. Artemisinin Metabolism and Its Synergism with Synthetic and Natural Products
13. What Lessons Can We Learn from the Tea?
14. Conclusions
- Sample Availability: Limited samples of a high-antioxidant ethanolic (70% ethanol) extract of A. annua are available from the corresponding author for chromatographic analysis.
References
- Mabeza, G.F.; Loyevsky, M.; Gordeuk, V.R.; Weiss, G. Iron chelation therapy for malaria: a review. Pharmacol. Therapeut. 1999, 81, 53–75. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromat. B 2004, 812, 85–99. [Google Scholar]
- Efferth, T.; Benakis, A.; Romero, M.R.; Tomicic, M.; Rauh, R.; Steinbach, D.; Häfer, R.; Stamminger, T.; Oesch, F.; Kaina, B.; Marschall, M. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Rad. Biol. Med. 2004, 37, 998–1009. [Google Scholar] [CrossRef]
- Erel, O.; Vural, H.; Aksoy, N.; Aslan, G.; Ulukanligil, M. Oxidative stress of platelets and thrombocytopenia in patients with vivax malaria. Clin. Biochem. 2001, 34, 341–344. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biologic. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Willcox, M.; Falquet, J.; Ferreira, J.F.S.; Gilbert, B.; Hsu, E.; Magalhães, P.M.; Plaizier-Vercammen, J.; Sharma, V.P.; Wright, C.W.; Yaode, W. Artemisia annua as an herbal tea for malaria. African J. Trad. Complem. Altern. Med. 2007, 4, 121–123. [Google Scholar]
- Rath, K.; Taxis, K.; Walz, G.; Gleiter, C.H.; Li, S.-M.; Heide, L. Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (annual wormwood). Am. J. Trop. Med. Hyg. 2004, 70, 128–132. [Google Scholar]
- Blanke, C.H.; Naisabha, G.B.; Balema, M.B.; Mbaruku, G.M.; Heide, L.; Muller, M.S. Herba Artemisiae annuae tea preparation compared to sulfadoxine-pyrimethamine in the treatment of uncomplicated falciparum malaria in adults: a randomized double-blind clinical trial. Trop. Doct. 2008, 38, 113–116. [Google Scholar] [CrossRef]
- Ittarat, W.; Pickard, A.L.; Rattanasinganchan, P.; Wilairatana, P.; Looareesuwan, S.; Emery, K.; Low, J.; Udomsangpetch, R.; Meshnick, S.R. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am. J. Trop. Med. Hyg. 2003, 68, 147–152. [Google Scholar]
- Price, R.; van Vugt, M.; Nosten, F.; Luxemburger, C.; Brockman, A.; Phaipun, L.; Chongsuphajaisiddhi, T.; White, N. Artesunate versus artemether for the treatment of recrudescent multidrug-resistant falciparum malaria. Am. J. Trop. Med. Hyg. 1998, 59, 883–888. [Google Scholar]
- Menard, D.; Matsika-Claquin, M.D.; Djalle, D.; Yapou, F.; Manirakiza, A.; Dolmazon, V.; Sarda, J.; Talarmin, A. Association of failures of seven-day courses of artesunate in a non-immune population in Bangui, Central African Republic with decreased sensitivity to Plasmodium falciparum. Am. J. Trop. Med. Hyg. 2005, 73, 616–621. [Google Scholar]
- Hsu, E. Reflections on the 'discovery' of the antimalarial qinghao. Brit. J. Clin. Pharmacol. 2006, 61, 666–670. [Google Scholar] [CrossRef]
- Quideau, S. Why bother with polyphenols. In Groupe Polyphenols: The International Society Dedicated to the Promotion of Research on Polyphenols; Bordeaux: France, 2009. [Google Scholar]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Comp. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chrom. A 2004, 1054, 95–111. [Google Scholar]
- George, T.W.; Niwat, C.; Waroonphan, S.; Gordon, M.H.; Lovegrove, J.A.; Paterson, E. Effect of chronic and acute fruit and vegetable juice consumption on cardiovascular disease risk factor. Acta Hort. 2009, 841, 201–206. [Google Scholar]
- Aggarwal, B.B. Targeting inflammatory pathways for chronic diseases by phytochemicals derived from spices, fruits, vegetables, and traditional remedies. Acta Hort. 2009, 841, 33–46. [Google Scholar]
- Luthria, D.L. Significance of sample preparation in developing analytical methodologies for accurate estimation of bioactive compounds in functional foods. J. Sci. Food Agric. 2006, 86, 2266–2272. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: an overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B.R., Jones, W.G., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1250–1318. [Google Scholar]
- Antolovich, M.; Prenzler, P.; Robards, K.; Ryan, D. Sample preparation in the determination of phenolic compounds in fruits. Analyst 2000, 125, 989–1009. [Google Scholar] [CrossRef]
- Escarpa, A.; Gonzalez, M.C. An overview of analytical chemistry of phenolic compounds in foods. Crit. Rev.Analyt. Chem. 2001, 31, 57–139. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Lai, J.-P.; Lim, Y.H.; Su, J.; Shen, H.-M.; Ong, C.N. Identification and characterization of major flavonoids and caffeoylquinic acids in three Compositae plants by LC/DAD-APCI/MS. J. Chrom. B 2007, 848, 215–225. [Google Scholar]
- Brown, G.D. Two new compounds from Artemisia annua. J. Nat. Prod. 1992, 55, 1756–1760. [Google Scholar] [CrossRef]
- Bhakuni, R.S.; Jain, D.C.; Sharma, R.P.; Kumar, S. Secondary metabolites of Artemisia annua and their biological activity. Curr. Sci. 2001, 80, 35–48. [Google Scholar]
- Elford, B.C.; Roberts, M.F.; Phillipson, J.D.; Wilson, R.J. Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 434–436. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Liersch, R.; Soicke, H.; Stehr, C. Tullner Formation of artemisinin in Artemisia annua during one vegetation period. Planta Med. 1986, 52, 387–390. [Google Scholar] [CrossRef]
- Luo, S.D.; B.M., N.; Hu, W.Y.; Xie, J.L. Studies on peroxides of Artemisia lamcea. J. Nat. Prod. 1991, 54, 573–575. [Google Scholar] [CrossRef]
- Marchese, J.A.; Broetto, F.; Ming, L.C.; Ducatti, C.; Rodella, R.A.; Ventrella, M.C.; Gomes, G.D.R.; de Franceschi, L. Carbon isotope composition and leaf anatomy as a tool to characterize the photosynthetic mechanism of Artemisia annua L. Braz. J. Plant Phys. 2005, 17, 187–190. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Laughlin, J.C.; Delabays, N.; Magalhães, P.M. Cultivation and genetics of Artemisia annua for increased production of the anti-malarial artemisinin. Plant Gen. Resourc. 2005, 3, 206–229. [Google Scholar] [CrossRef]
- Baraldi, R.; Isacchi, B.; Predieri, S.; Marconi, G.; Vincieri, F.F.; Bilia, A.R. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem. Syst. Ecol. 2008, 36, 340–348. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Simon, J.E.; Janick, J. Developmental studies of Artemisia annua: Flowering and artemisinin production under greenhouse and field conditions. Planta Med. 1995, 61, 167–170. [Google Scholar] [CrossRef]
- Ferreira, J.F.S. Seasonal and post-harvest accumulation of artemisinin, artemisinic acid, and dihydroartemisinic acid in three accessions of Artemisia annua cultivated in West Virginia, USA. Planta Med. 2008, 74, 310–311. [Google Scholar]
- Wallaart, T.E.; Pras, N.; Quax, W.J. Seasonal variations of artemisinin and its biosynthetic precursors in tetraploid Artemisia annua plants compared with diploid wild-type. Planta Med. 1999, 65, 723–728. [Google Scholar] [CrossRef]
- Bilia, A.R.; Magalhães, P.M.; Bergonzi, M.C.; Vincieria, F.F. Simultaneous analysis of artemisinin and flavonoids of several extracts of Artemisia annua L. obtained from a commercial sample and a selected cultivar. Phytomedicine 2006, 13, 487–493. [Google Scholar] [CrossRef]
- Ferreira, J.F.S. Artemisia species in small ruminant production: their potential antioxidant and anthelmintic effects. In Appalachian Workshop and Research Update: Improving small ruminant grazing practices; Morales, M., Ed.; Mountain State University/USDA: Beaver, WV, USA, 2009; pp. 53–70. [Google Scholar]
- Ro, D.-K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; Chang, M.C.Y.; Withers, S.T.; Shiba, Y.; Sarpong, R.; Keasling, J.D. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef]
- Wallaart, T.E.; Pras, N.; Beekman, A.C.; Quax, W.J. Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: Proof for the existence of chemotypes. Planta Med. 2000, 66, 57–62. [Google Scholar]
- Le Marchand, L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of flavonoids and lung cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef]
- Walle, T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin. Cancer Biol. 2007, 17, 354–362. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids increase the intracellular glutathione level by transactivation of the [gamma]-glutamylcysteine synthetase catalytical subunit promoter. Free Rad. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyoshi, N. Electrophiles in foods: The current status of isothiocyanates and their chemical biology. Biosci. Biotechnol. Biochem. 2010, 74, 242–255. [Google Scholar] [CrossRef]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.C.W.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277–283. [Google Scholar]
- Yokoyama, A.; Sakakibara, H.; Crozier, A.; Kawai, Y.; Matsui, A.; Terao, J.; Kumazawa, S.; Shimoi, K. Quercetin metabolites and protection against peroxynitrite-induced oxidative hepatic injury in rats. Free Rad. Res. 2009, 43, 913–921. [Google Scholar] [CrossRef]
- Suri, S.; Liu, X.H.; Rayment, S.; Hughes, D.A.; Kroon, P.A.; Needs, P.W.; Taylor, M.A.; Tribolo, S.; Wilson, V.G. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Brit. J. Pharmacol. 2009, 159, 566–575. [Google Scholar]
- Liao, H.; Banbury, L.K.; Leach, D.N. Antioxidant activity of 45 Chinese herbs and the relationship with their TCM characteristics. eCAM 2008, 5, 429–434. [Google Scholar]
- Willcox, M.; Falquet, J.; Ferreira, J.F.S.; Gilbert, B.; Hsu, E.; Magalhães, P.M.; Plaizier-Vercammen, J.; Sharma, V.P.; Wright, C.W.; Yaode, W. Artemisia annua as a herbal tea for malaria. Afr. J. Trad. CAM 2007, 4, 121–123. [Google Scholar]
- Monbrison, F.; Maitrejean, M.; Latour, C.; Bugnazet, F.; Peyron, F.; Barron, D.; Picot, S. In vitro antimalarial activity of flavonoid derivatives dehydrosilybin and 8-(1;1)-DMA-kaempferide. Acta Trop. 2006, 97, 102–107. [Google Scholar] [CrossRef]
- Silveira, P.; Vashist, U.; Cabral, A.; Amaral, K.; Soares, G.; Dagosto, M. Effect of rutin and chloroquine on White Leghorn chickens infected with Plasmodium (Bennettinia) juxtanucleare. Trop. Anim. Health Produc. 2009.
- Lehane, A.; Saliba, K. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res. Notes 2008, 1, 26. [Google Scholar] [CrossRef]
- Willcox, M. Artemisia species: from traditional medicines to modern antimalarials-and back again. JACM 2009, 15, 101–109. [Google Scholar]
- Hollman, P.C.H.; Gaag, M.V.D.; Mengelers, M.J.B.; Van Trijp, J.M.P.; De Vries, J.H.M.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Rad. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef]
- Bilia, A.R.; Lazari, D.; Messori, L.; Taglioli, V.; Temperini, C.; Vincieri, F.F. Simple and rapid physico-chemical methods to examine action of antimalarial drugs with hemin: Its application to Artemisia annua constituents. Life Sci. 2002, 70, 769–778. [Google Scholar] [CrossRef]
- Liu, K.C.-S.; Yang, S.-L.; Roberts, M.E.; Elford, B.C.; Phillipson, J.D. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992, 11, 637–640. [Google Scholar]
- Bilia, A.R.; Sannella, A.R.; Vincieri, F.F.; Messori, L.; Casini, A.; Gabbiani, C.; Severini, C.; Majori, G. Antiplasmodial effects of a few selected natural flavonoids and their modulation of artemisinin activity. Nat. Prod. Commun. 2008, 3, 1999–2002. [Google Scholar]
- Lu, J.; Papp, L.V.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar] [CrossRef]
- Krnajski, Z.; Gilberger, T.W.; Walter, R.D.; Cowman, A.F.; Müller, S. Thioredoxin reductase is essential for the survival of Plasmodium falciparum erythrocytic stages. J. Biolog. Chem. 2002, 277, 25970–25975. [Google Scholar]
- Kun, J.F.J.; Hibbs, A.R.; Saul, A.; McColl, D.J.; Coppel, R.L.; Anders, R.F. A putative Plasmodium falciparum exported serine/threonine protein kinase. Mol. Biochem. Parasitol. 1997, 85, 41–51. [Google Scholar] [CrossRef]
- Kale, A.; Gawande, S.; Kotwal, S. Cancer phytotherapeutics: role for flavonoids at the cellular level. Phytoth. Res. 2008, 22, 567–577. [Google Scholar] [CrossRef]
- Arts, I.C.W. A review of the epidemiological evidence on tea, flavonoids, and lung cancer. J. Nutr. 2008, 138, 1561–1566. [Google Scholar]
- Le Marchand, L. Cancer preventive effects of flavonoids-a review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar]
- Lai, H.; Singh, N.P. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995, 91, 41–46. [Google Scholar] [CrossRef]
- Moore, J.C.; Lai, H.; Li, J.-R.; Ren, R.-L.; McDougall, J.A.; Singh, N.P.; Chou, C.-K. Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett. 1995, 98, 83–87. [Google Scholar]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar]
- Singh, N.P.; Lai, H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001, 70, 49–56. [Google Scholar] [CrossRef]
- Lai, H.; Sasaki, T.; Singh, N.P.; Messay, A. Effects of artemisinin-tagged holotrasferrin on cancer cells. Life Sci. 2005, 76, 1267–1279. [Google Scholar] [CrossRef]
- Nakase, I.; Gallis, B.; Takatani-Nakase, T.; Oh, S.; Lacoste, E.; Singh, N.P.; Goodlett, D.R.; Tanaka, S.; Futaki, S.; Lai, H.; Sasaki, T. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009, 274, 290–298. [Google Scholar] [CrossRef]
- Oh, S.; Kim, B.J.; Singh, N.P.; Lai, H.; Sasaki, T. Synthesis and anti-cancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Lett. 2009, 274, 33–39. [Google Scholar] [CrossRef]
- Firestone, G.L.; Sundar, S.N. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev. Mol. Med. 2009, 11, 1–15. [Google Scholar] [CrossRef]
- Beutler, J.A.; Hamel, E.; Vlietinck, A.J.; Haemers, A.; Rajan, P.; Roitman, J.N.; Cardellina II, J.H.; Boyd, M.R. Structure-Activity Requirements for Flavone Cytotoxicity and Binding to Tubulin. J. Med. Chem. 1998, 41, 2333–2338. [Google Scholar] [CrossRef]
- Henrich, C.J.; Bokesch, H.R.; Dean, M.; Bates, S.E.; Robey, R.W.; Goncharova, E.I.; Wilson, J.A.; McMahon, J.B. A yigh-throughput cell-based assay for inhibitors of ABCG2 activity. J. Biomolec. Screen. 2006, 11, 176–183. [Google Scholar]
- Zhou, L.; Liu, P.; Chen, B.; Wang, Y.; Wang, X.; Internati, M.C.; Wachtel, M.S.; Frezza, E.E. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008, 28, 1119–1127. [Google Scholar]
- Son, Y.-G.; Kim, E.H.; Kim, J.Y.; Kim, S.U.; Kwon, T.K.; Yoon, A.-R.; Yun, C.-O.; Choi, K.S. Silibinin Sensitizes Human Glioma Cells to TRAIL-Mediated Apoptosis via DR5 Up-regulation and Down-regulation of c-FLIP and Survivin. Cancer Res. 2007, 67, 8274–8284. [Google Scholar]
- Imai, Y.; Tsukahara, S.; Asada, S.; Sugimoto, Y. Phytoestrogens/Flavonoids Reverse Breast Cancer Resistance Protein/ABCG2-Mediated Multidrug Resistance. Cancer Res. 2004, 64, 4346–4352. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Agarwal, P.; Glode, L.M.; Agarwal, R. Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. Int. J. Cancer 2003, 106, 699–705. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, E.H.; Park, S.S.; Lim, J.H.; Kwon, T.K.; Choi, K.S. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J. Cell Biochem. 2008, 105, 1386–1398. [Google Scholar] [CrossRef]
- Chan, M.M.; Fong, D. Overcoming ovarian cancer drug resistance with phytochemicals and other compounds. Prog. Cancer Drug Resist. Res. 2007, 1–28. [Google Scholar]
- Zanini, C.; Giribaldi, G.; Mandili, G.; Carta, F.; Crescenzio, N.; Bisaro, B.; Doria, A.; Foglia, L.; Cordero di Montezemolo, L.; Timeus, F.; Turrini, F. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and Ewing's sarcoma cell lines. J. Neurochem. 2007, 103, 1344–1354. [Google Scholar] [CrossRef]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J. Chemother. 2005, 17, 86–95. [Google Scholar]
- Sliutz, G.; Karlseder, J.; Tempfer, C.; Orel, L.; Holzer, G.; Simon, M.M. Drug resistance against gemcitabine and topotecan mediated by constitutive hsp70 overexpression in vitro: Implication of quercetin as sensitizer in chemotherapy. Brit. J. Cancer 1996, 74, 172–177. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Jernas, K.; Krol, W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int. J. Mol. Sci. 2008, 9, 56–64. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Sakai, T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol. Cancer Ther. 2006, 5, 945–951. [Google Scholar] [CrossRef]
- Long, X.; Fan, M.; Bigsby, R.M.; Nephew, K.P. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol. Cancer Ther. 2008, 7, 2096–2108. [Google Scholar]
- Chiang, C.-T.; Way, T.-D.; Lin, J.-K. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21WAF1/CIP1 expression with rapamycin. Mol. Cancer Ther. 2007, 6, 2127–2138. [Google Scholar] [CrossRef]
- Du, G.-J.; Song, Z.-H.; Lin, H.-H.; Han, X.-F.; Zhang, S.; Yang, Y.-M. Luteolin as a glycolysis inhibitor offers superior efficacy and lesser toxicity of doxorubicin in breast cancer cells. Biochim. Biophys. Acta Res. Commun. 2008, 372, 497–502. [Google Scholar]
- Shi, R.; Huang, Q.; Zhu, X.; Ong, Y.-B.; Zhao, B.; Lu, J.; Ong, C.-N.; Shen, H.-M. Luteolin sensitizes the anticancer effect of cisplatin via c-Jun NH2-terminal kinase-mediated p53 phosphorylation and stabilization. Mol. Cancer Ther. 2007, 6, 1338–1347. [Google Scholar] [CrossRef]
- Shi, R.-X.; Ong, C.-N.; Shen, H.-M. Protein Kinase C Inhibition and X-Linked Inhibitor of Apoptosis Protein Degradation Contribute to the Sensitization Effect of Luteolin on Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Cancer Cells. Cancer Res. 2005, 65, 7815–7823. [Google Scholar]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Herold-Mende, C.; von Deimling, A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol. Cancer Ther. 2008, 7, 3566–3574. [Google Scholar] [CrossRef]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef]
- Michelis, F.; Tiligada, E.; Skaltsa, H.; Lazari, D.; Skaltsounis, A.-L.; Delitheos, A. Effects of the flavonoid pilloin isolated from Marrubium cylleneum on mitogen-induced lymphocyte transformation. Pharmaceut. Biol. 2002, 40, 245–248. [Google Scholar] [CrossRef]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar]
- Li, Z.-D.; Hu, X.-W.; Wang, Y.-T.; Fang, J. Apigenin inhibits proliferation of ovarian cancer A2780 cells through Id1. FEBS Lett. 2009, 582, 1999–2003. [Google Scholar]
- Melstrom, L.G.; Salabat, M.R.; Ding, X.-Z.; Milam, B.M.; Strouch, M.; Pelling, J.C.; Bentrem, D.J. Apigenin Inhibits the GLUT-1 Glucose Transporter and the Phosphoinositide 3-Kinase/Akt Pathway in Human Pancreatic Cancer Cells. Pancreas 2008, 37, 426–431. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Woelfle, U.; Schempp, C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef]
- Yoo, D.R.; Jang, Y.H.; Jeon, Y.K.; Kim, J.Y.; Jeon, W.; Choi, Y.J.; Nam, M.J. Proteomic identification of anti-cancer proteins in luteolin-treated human hepatoma Huh-7 cells. Cancer Lett. 2009, 282, 48–54. [Google Scholar] [CrossRef]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef]
- Tezuka, Y.; Stampoulis, P.; Banskota, A.H.; Awale, S.; Tran, K.Q.; Saiki, I.; Kadota, S. Constituents of the vietnamese medicinal plant Orthosiphon stamineus. Chem. Pharm. Bull. 2000, 48, 1711–1719. [Google Scholar] [CrossRef]
- Dobberstein, R.H.; Tin-Wa, M.; Fong, H.H.S.; Crane, F.A.; Farnsworth, N.R. Flavonoid constituents from Eupatorium altissimum L. (Compositae). J. Pharm. Sci. 1977, 66, 600–602. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Arroo, R.R.J.; F., H.J.; Surichan, S.; A., P.G. Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. Breast Cancer Res. 2008, 10, R39. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Li, N.; Arroo, R.R.J. The methoxylated flavones eupatorin and cirsiliol induce CYP1 enzyme expression in MCF7 cells. J. Nat. Prod. 2009, 72, 1390–1394. [Google Scholar] [CrossRef]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef]
- Sheng, X.; Sun, Y.; Yin, Y.; Chen, T.; Xu, Q. Cirsilineol inhibits proliferation of cancer cells by inducing apoptosis via mitochondrial pathway. J. Pharm. Pharmacol. 2008, 60, 1523–1529. [Google Scholar]
- Beutler, J.A.; Hamel, E.; Vlietinck, A.J.; Haemers, A.; Rajan, P.; Roitman, J.N.; Cardellina II, J.H.; Boyd, M.R. Structure-activity requirements for flavone cytotoxicity and binding to tubulin. J. Med. Chem. 1998, 41, 2333–2338. [Google Scholar] [CrossRef]
- Bai, N.; Zhou, Z.; Zhu, N.; Zhang, L.; Quan, Z.; He, K.; Zheng, Q.Y.; Ho, C.-T. Antioxidant flavonoids from the flower of Inula britannica. J. Food Lip. 2005, 12, 141–149. [Google Scholar] [CrossRef]
- Henrich, C.J.; Bokesch, H.R.; Dean, M.; Bates, S.E.; Robey, R.W.; Goncharova, E.I.; Wilson, J.A.; McMahon, J.B. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J. Biomol. Screen. 2006, 11, 176–183. [Google Scholar]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997, 112, 127–133. [Google Scholar] [CrossRef]
- Rodgers, E.H.; Grant, M.H. The effect of the flavonoids quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chemico-Biol. Interact. 1998, 116, 213–228. [Google Scholar] [CrossRef]
- Wenzel, U.; Herzog, A.; Kuntz, S.; Daniel, H. Protein expression profiling identifies molecular targets of quercetin as a major dietary flavonoid in human colon cancer cells. Proteomics 2004, 4, 2160–2174. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; Bonanno, G.; Vencenzo, R.D.; Piantelli, M.; Mancuso, S. Synergistic Antiproleferative Activity of Quercetin and Cisplatin on Ovarian Cancer Cell Growth. Anti-Cancer Drugs 1990, 1, 45–48. [Google Scholar] [CrossRef]
- Ranelletti, F.O.; Ricci, R.; Larocca, L.M.; Maggiano, N.; Capelli, A.; Scambia, G.; Benedetti-Panici, P.; Mancuso, S.; Rumi, C.; Piantelli, M. Growth-inhibitory effect of quercetin and presence of type-II estrogen-binding sites in human colon-cancer cell lines and primary colorectal tumors. Int. J. Cancer. 2006, 50, 486–492. [Google Scholar]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar]
- Zheng, G.Q. Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med. 1994, 60, 54–57. [Google Scholar] [CrossRef]
- Holder, H.; Zemskova, M.; Zhang, C.; Tabrizizad, M.; Bremer, R.; Neidigh, J.W.; Lilly, M.B. Characterization of a potent and selective small-molecule inhibitor of the PIM1 kinase. Mol. Cancer Ther. 2007, 6, 163–172. [Google Scholar] [CrossRef]
- Knockaert, M.; Greengard, P.; Meijer, L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol. Sci. 2002, 23, 417–425. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Jones, W.P.; Mi, Q.; Rachman, I.; Riswan, S.; Kardono, L.B.S.; Chai, H.-B.; Farnsworth, N.R.; Cordell, G.A.; Swanson, S.M.; Cassady, J.M.; Kinghorn, A.D. Cytotoxic clerodane diterpenoids from the leaves of Premna tomentosa. Phytochemistry 2006, 67, 1243–1248. [Google Scholar]
- Ono, M.; Yanaka, T.; Yamamoto, M.; Ito, Y.; Nohara, T. New diterpenes and norditerpenes from the fruits of Vitex rotundifolia. J. Nat. Prod. 2002, 65, 537–541. [Google Scholar] [CrossRef]
- Kobayakawa, J.; Sato-Nishimori, F.; Moriyasu, M.; Matsukawa, Y. G2-M arrest and antimitotic activity mediated by casticin, a flavonoid isolated from Viticis Fructus (Vitex rotundifolia Linne fil.). Cancer Lett. 2004, 208, 59–64. [Google Scholar] [CrossRef]
- Haidara, K.; Zamir, L.; Shi, Q.-W.; Batist, G. The flavonoid casticin has multiple mechanisms of tumor cytotoxicity action. Cancer Lett. 2006, 242, 180–190. [Google Scholar] [CrossRef]
- Svensson, U.S.; Sandstrom, R.; Carlborg, O.; Lennernas, H.; Ashton, M. High in situ rat intestinal permeability of artemisinin unaffected by multile dosing and with no evidence of P-glycoprotein involvement. Drug Metab. Disp. 1999, 27, 227–232. [Google Scholar]
- Li, S.; Pan, M.-H.; Lai, C.-S.; Lo, C.-Y.; Dushenkov, S.; Ho, C.-T. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg. Med. Chem. 2007, 15, 3381–3389. [Google Scholar] [CrossRef]
- Arisawa, M.; Hayashi, T.; Shimizu, M.; Morita, N.; Bai, H.; Kuze, S.; Ito, Y. Isolation and cytotoxicity of two new flavonoids from Chrysosplenium grayanum and related flavonols. J. Nat. Prod. 1991, 54, 898–901. [Google Scholar] [CrossRef]
- Arisawa, M.; Shimizu, M.; Satomi, Y.; Nishino, A.; Nishino, H.; Iwashima, A. Inhibition of tumor-promoter-enhanced 32Pi-incorporation into cellular phospholipids by flavonols from genus Chrysosplenium. Phytother. Res. 1995, 9, 222–224. [Google Scholar] [CrossRef]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Cytotoxicity of plant flavonoids against HeLa cells. Phytochemistry 1988, 27, 1017–1020. [Google Scholar]
- Mutoh, M.; Takahashi, M.; Fukuda, K.; Komatsu, H.; Enya, T.; Matsushima-Hibiya, Y.; Mutoh, H.; Sugimura, T.; Wakabayashi, K. Suppression by flavonoids of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells: structure-activity relationship. Jpn. J. Cancer Res. 2000, 91, 686–691. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative Activities of Citrus Flavonoids against Six Human Cancer Cell Lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Jeong, K.-W.; Kim, W.; Heo, Y.S.; Kim, Y. Binding Models of Flavonols to Human Vascular Endothelial Growth Factor Receptor 2. Bull. Korean Chem. Soc. 2009, 30, 2083–2086. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.-H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Kang, G.-Y.; Lee, E.-R.; Kim, J.-H.; Jung, J.W.; Lim, J.; Kim, S.K.; Cho, S.-G.; Kim, K.P. Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. Eur. J. Pharmacol. 2009, 611, 17–21. [Google Scholar] [CrossRef]
- Chung, S.Y.; Jang, D.S.; Han, A.-R.; Jang, J.O.; Kwon, Y.; Seo, E.-K.; Lee, H.J. Modulation of P-glycoprotein-mediated resistance by kaempferol derivatives isolated from Zingiber zerumbet. Phytother. Res. 2007, 21, 565–569. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef]
- Labbe, D.; Provencal, M.; Lamy, S.; Boivin, D.; Gingras, D.; Beliveau, R. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J. Nutr. 2009, 139, 646–652. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba extract kaempferol Inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Romero, J.R.; Chattopadhyay, N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol. Cell. Endocrinol. 2008, 287, 57–64. [Google Scholar] [CrossRef]
- Leung, H.W.-C.; Lin, C.-J.; Hour, M.-J.; Yang, W.-H.; Wang, M.-Y.; Lee, H.-Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Tox. 2007, 45, 2005–2013. [Google Scholar] [CrossRef]
- Teng, B.-S.; Lu, Y.-H.; Wang, Z.-T.; Tao, X.-Y.; Wei, D.-Z. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol. Res. 2006, 54, 186–194. [Google Scholar] [CrossRef]
- Ma, G.; Yang, C.; Qu, Y.; Wei, H.; Zhang, T.; Zhang, N. The flavonoid component isorhamnetin in vitro inhibits proliferation and induces apoptosis in Eca-109 cells. Chemico-Biol. Interact. 2007, 167, 153–160. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, H.-J.; Lee, E.-O.; Ko, S.-G.; Bae, H.-S.; Kim, C.-H.; Ahn, K.-S.; Lu, J.; Kim, S.-H. Mitochondria-cytochrome C-caspase-9 cascade mediates isorhamnetin-induced apoptosis. Cancer Lett. 2008, 270, 342–353. [Google Scholar] [CrossRef]
- De Leo, M.; Braca, A.; Sanogo, R.; Cardile, V.; DeTommasi, N.; Russo, A. Antiproliferative activity of Pteleopsis suberosa leaf extract and its flavonoid components in human prostate carcinoma cells. Planta Med. 2006, 72, 604–610. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Merfort, I.; Passreiter, C.M.; Schmidt, T.J.; Willuhn, G.; van Uden, W.; Pras, N.; Kampinga, H.H.; Konings, A.W.T. Cytotoxicity of flavonoids and sesquiterpene lactones from Arnica species against the GLC4 and the COLO 320 cell lines. Planta Med. 1994, 60, 434–437. [Google Scholar] [CrossRef]
- Matsubara, K.; Ishihara, K.; Mizushina, Y.; Mori, M.; Nakajima, N. Anti-angiogenic activity of quercetin and its derivatives. Lett. Drug Design Disc. 2004, 1, 329–333. [Google Scholar] [CrossRef]
- Wright, C.W.; Warhurst, D.C. The mode of action of artemisinin and its derivatives. In Artemisia; Wright, C.W., Ed.; Taylor & Francis Inc.: New York, NY, USA, 2002; pp. 249–288. [Google Scholar]
- Hein, E.-M.; Rose, K.; van't Slot, G.; Friedrich, A.W.; Humpf, H.-U. Deconjugation and degradation of flavonol glycosides by pig cecal microbiota characterized by fluorescence in situ hybridization (FISH). J. Agric. Food Chem. 2008, 56, 2281–2290. [Google Scholar]
- Konishi, Y.; Kobayashi, S.; Shimizu, M. Tea polyphenols inhibit the transport of dietary phenolic acids mediated by the monocarboxylic acid transporter (MCT) in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2003, 51, 7296–7302. [Google Scholar] [CrossRef]
- Van't Slot, G.; Humpf, H.-U. Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Abrahamse, S.L.; Kloots, W.J.; van Amelsvoort, J.M.M. Absorption, distribution, and secretion of epicatechin and quercetin in the rat. Nutr. Res. 2005, 25, 305–317. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243–255. [Google Scholar]
- Svensson, U.S.H.; Ashton, M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br. J. Clin. Pharmacol. 1999, 48, 528–535. [Google Scholar] [CrossRef]
- Batty, K.T.; Thu, L.T.A.; Davis, T.M.E.; Ilett, K.F.; Mai, T.X.; Hung, N.C.; Tien, N.P.; Powell, S.M.; Thien, H.V.; Binh, T.Q.; Kim, N.V. A pharmacokinetic and pharmacodynamic study of intravenous vs. oral artesunate in uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 1998, 45, 123–129. [Google Scholar]
- Keiser, J.; Gruyer, M.-S.; Perrottet, N.; Zanolari, B.; Mercier, T.; Decosterd, L. Pharmacokinetic parameters of artesunate and dihydroartemisinin in rats infected with Fasciola hepatica. J. Antimicrob. Chemother. 2009, 63, 543–549. [Google Scholar] [CrossRef]
- Nandakumar, D.N.; Nagaraj, V.A.; Vathsala, P.G.; Rangarajan, P.; Padmanaban, G. Curcumin-Artemisinin Combination Therapy for Malaria. Antimicrob. Agents Chemother. 2006, 50, 1859–1860. [Google Scholar] [CrossRef]
- Lai, J.-P.; Lim, Y.H.; Su, J.; Shen, H.-M.; Ong, C.N. Identification and characterization of major flavonoids and caffeoylquinic acids in three Compositae plants by LC/DAD-APCI/MS. J. Chrom. B 2007, 848, 215–225. [Google Scholar] [CrossRef]
- Dupuy, J.; Larrieu, G.; Sutra, J.F.; Lespine, A.; Alvinerie, M. Enhancement of moxidectin bioavailability in lamb by a natural flavonoid: quercetin. Vet. Parasitol. 2003, 112, 337–347. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Scriven, L.N.; Tegos, G.; Lewis, K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002, 68, 1140–1141. [Google Scholar] [CrossRef]
- Sannella, A.R.; Messori, L.; Casini, A.; Vincieri, F.F.; Bilia, A.R.; Majori, G.; Severini, C. Antimalarial properties of green tea. Biochem. Biophys. Res. Commun. 2007, 353, 177–181. [Google Scholar] [CrossRef]
- Gupta, S.; Thapar, M.M.; Wernsdorfer, W.H.; Bjorkman, A. In vitro interactions of artemisinin with atovaquone, quinine, and mefloquine against Plasmodium falciparum. Antimicrob. Agents Chemother. 2002, 46, 1510–1515. [Google Scholar] [CrossRef]
- de Oliveira, T.C.; Silva, D.A.O.; Rostkowska, C.; Béla, S.R.; Ferro, E.A.V.; Magalhães, P.M.; Mineo, J.R. Toxoplasma gondii: effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Experim. Parasitol. 2009, 122, 233–241. [Google Scholar] [CrossRef]
- van Agtmael, M.A.; Gupta, V.; van der Wösten, T.H.; Rutten, J.P.B.; van Boxtel, C.J. Grapefruit juice increases the bioavailability of artemether. Eur. J. Clin. Pharmacol. 1999, 55, 405–410. [Google Scholar] [CrossRef]
- El-Lakkany, N.M.; Seif el-Din, S.H.; Badawy, A.A.; Ebeid, F.A. Effect of artemether alone and in combination with grapefruit juice on hepatic drug-metabolising enzymes and biochemical aspects in experimental Schistosoma mansoni. Int. J. Parasitol. 2004, 34, 1405–1412. [Google Scholar] [CrossRef]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal modulation of p-glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar] [CrossRef]
- Yarnell, E.; Abascal, K. Interaction of herbal constituents with cytochrome P450 enzymes. Altern. Complem. Ther. 2007, 239–247. [Google Scholar] [CrossRef]
- Soh, P.N.; Witkowski, B.; Olagnier, D.; Nicolau, M.-L.; Garcia-Alvarez, M.-C.; Berry, A.; Benoit-Vical, F. In vitro and in vivo properties of ellagic acid in malaria treatment. Antimicrob. Agents Chemother. 2009, 53, 1100–1106. [Google Scholar] [CrossRef]
- Ekong, R.; Warhurst, D.C. Synergism between arteether and mefloquine or quinine in a multidrug-resistant strain of Plasmodium falciparum in vitro. Trans. Royal Soc. Trop. Med. Hyg. 1990, 84, 757–758. [Google Scholar] [CrossRef]
- Akoachere, M.; Buchholz, K.; Fischer, E.; Burhenne, J.; Haefeli, W.E.; Schirmer, R.H.; Becker, K. In vitro assessment of methylene blue on chloroquine-sensitive and -resistant Plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob. Agents Chemother. 2005, 49, 4592–4597. [Google Scholar] [CrossRef]
- WHO. WHO announces pharmaceutical companies agree to stop marketing single-drug artemisinin malaria pills. Available online: http://www.who.int/mediacentre/news/releases/2006/pr23/en/print.html/ (accessed on 29 April 2010).
- Hsu, E. The history of qing hao in the Chinese materia medica. Transac. Royal Soc. Trop. Med. Hyg. 2006, 100, 505–508. [Google Scholar] [CrossRef]
- Mueller, M.S.; Runyambo, N.; Wagner, I.; Borrmann, S.; Dietz, K.; Heide, L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans. Royal Soc. Trop. Med. Hyg. 2004, 98, 318–321. [Google Scholar] [CrossRef]
- Mueller, M.S.; Karhagomba, I.B.; Hirt, H.M.; Wemakor, E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects. J. Ethnopharm. 2000, 73, 487–493. [Google Scholar] [CrossRef]
- Hollman, P.C. Evidence for health benefits of plant phenols: Local or systemic effects. J. Sci. Food Agric. 2001, 81, 842–852. [Google Scholar] [CrossRef]
- Atemnkeng, M.A.; Chimanuka, B.; Dejaegher, B.; Heyden, Y.V.; Plaizier-Vercammen, J. Evaluation of Artemisia annua infusion efficacy for the treatment of malaria in Plasmodium chabaudi chabaudi infected mice. Exper. Parasitol. 2009, 122, 344–348. [Google Scholar] [CrossRef]
- Wright, C.W.; Linley, P.A.; Brun, R.; Wittlin, S.; Hsu, E. Ancient chinese methods are remarkably effective for the preparation of artemisinin-rich extracts of qing hao with potent antimalarial activity. Molecules 2010, 15, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Dueñas, M.; González-Manzano, S.; González-Paramás, A.; Santos-Buelga, C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar] [CrossRef]
- Kinghorn, D.A.; Farnsworth, N.; Soejarto, D.; Cordell, G.; Swanson, S.; Pezzuto, J.; Wani, M.; Wall, M.; Oberlies, N.H.; Kroll, D.; Kramer, R.; Rose, W.; Vite, G.; Fairchild, C.; Peterson, R.; Wild, R. Novel strategies for the discovery of plant-derived anticancer agents. Pharm. Biol. 2003, 41, 53–67. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135-3170. https://doi.org/10.3390/molecules15053135
Ferreira JFS, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules. 2010; 15(5):3135-3170. https://doi.org/10.3390/molecules15053135
Chicago/Turabian StyleFerreira, Jorge F.S., Devanand L. Luthria, Tomikazu Sasaki, and Arne Heyerick. 2010. "Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer" Molecules 15, no. 5: 3135-3170. https://doi.org/10.3390/molecules15053135
APA StyleFerreira, J. F. S., Luthria, D. L., Sasaki, T., & Heyerick, A. (2010). Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules, 15(5), 3135-3170. https://doi.org/10.3390/molecules15053135