Antimicrobial Prospect of Newly Synthesized 1,3-Thiazole Derivatives
Abstract
:1. Introduction

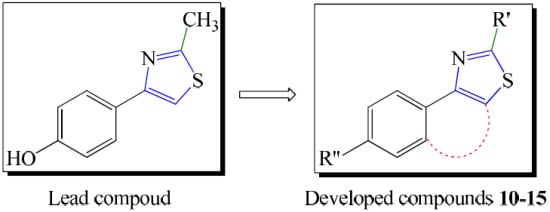
2. Results and Discussion
2.1. Chemistry: General Procedures for Synthesis

2.2. Biological Activity
| MIC µg/mL | Lipophilicity | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Structural formula | S. aureus | E. coli | A. niger | Log P | Clog P ** | ||
| Lead * |  | 150 * | 200 * | 150 * | 2.68 | 2.89 | ||
| 10 |  | 200 | 200 | 200 | 3.41 | 3.20 | ||
| 11 |  | 150 | 200 | 150 | 4.65 | 4.24 | ||
| 12 |  | 125 | 150 | 125 | 4.45 | 4.24 | ||
| 13 |  | 50 | 50 | 75 | 3.86 | 4.07 | ||
| 14 |  | 50 | 75 | 50 | 3.83 | 4.05 | ||
| 15 |  | 125 | 125 | 125 | 2.98 | 3.14 | ||
| Ofloxacin | 10 | 12.5 | -- | -- | -- | |||
| Ketoconazole | -- | -- | 12.5 | -- | -- | |||
2.3. Lipophilicity and QSAR

3. Experimental
3.1. General
3.1.1. 2-Ethyl-4-(4-methoxyphenyl)thiazole (4)
3.1.2. 4-(4-Methoxyphenyl)-2-phenylthiazole (5)
3.1.3. 2-(4-Methoxyphenyl)-4-phenylthiazole (6)
3.1.4. 2-(4-Methoxyphenyl)benzo[d]thiazole (7)
3.1.5. 2-(3-Methoxyphenyl)benzo[d]thiazole (8)
3.1.6. 2-(3,4-Dimethoxyphenyl)benzo[d]thiazole (9)
3.2. General Procedure for Ether Cleavage
3.2.1. 2-Ethyl-4-(4-hydroxyphenyl)thiazole (10)
3.2.2. 4-(4-Hydroxyphenyl)-2-phenylthiazole (11)
3.2.3. 2-(4-Hydroxyphenyl)-4-phenylthiazole (12)
3.2.4. 2-(4-Hydroxyphenyl)benzo[d]thiazole (13)
3.2.5. 2-(3-Hydroxyphenyl)benzo[d]thiazole (14)
3.2.6. 2-(3,4-Dihydroxyphenyl)benzo[d]thiazole (15)
3.3. Partition Coffeicient Determination
3.4. Antimicrobial Activity
4. Conclusions
Conflict of Interest
Acknowledgments
References
- Bayles, K.W. The bactericidal action of penicillin: New clues to an unsolved mystery. Trends Microbiol. 2000, 8, 274–278. [Google Scholar] [CrossRef]
- Carratala, J.; Alcaide, F.; Fernandez, S.A. Bacteremia due to viridans streptococci that are highly resistant to penicillin: Increase among neutropenic patients with cancer. Clin. Infect. Dis. 1995, 20, 1169–1173. [Google Scholar] [CrossRef]
- Sogn, D.D.; Evans, R.; Shepherd, G.M. Results of the National Institute of Allergy and Infectious Diseases Collaborative Clinical Trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch. Intern. Med. 1992, 152, 1025–1032. [Google Scholar] [CrossRef]
- Haas, D.W.; Stratton, C.W.; Griffin, J.P.; Weeks, L.; Alls, S.C. Diminished Activity of Ceftizoxime in Comparison to Cefotaxime and Ceftriaxone Against Streptococcus pneumoniae. Clin. Infect. Dis. 1995, 20, 671–676. [Google Scholar] [CrossRef]
- John, C.C. Treatment Failure with Use of a Third-Generation Cephalosporin for Penicillin-Resistant Pneumococcal Meningitis: Case Report and Review. Clin. Infect. Dis. 1994, 18, 188–193. [Google Scholar] [CrossRef]
- Schaad, U.B.; Suter, S.; Gianella, A.B. A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meningitis in children. N. Engl. J. Med. 1990, 322, 141–147. [Google Scholar] [CrossRef]
- Bradshaw, T.D.; Westwell, A.D. The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate. Curr. Med. Chem. 2004, 11, 1009–1021. [Google Scholar] [CrossRef]
- Hutchinson, I.; Jennings, S.A.; Vishnuvajjala, B.R.; Westwell, A.D.; Stevens, M.F.G. Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J. Med. Chem. 2002, 45, 744–747. [Google Scholar] [CrossRef]
- Hutchinson, I.; Chua, M.S.; Browne, H.L.; Trapani, V.; Bradshaw, T.D.; Westwell, A.D.; Stevens, M.F.G. Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2- (4-aminophenyl)benzothiazoles. J. Med. Chem. 2001, 44, 1446–1455. [Google Scholar] [CrossRef]
- Kashiyama, E.; Hutchinson, I.; Chua, M.S.; Stinson, S.F.; Phillips, L.R.; Kaur, G.; Sausville, E.A.; Bradshaw, T.D.; Westwell, A.D.; Stevens, M.F.G. Antitumor benzothiazoles. 8. Synthesis, metabolic formation, and biological properties of the C- and N-oxidation products of antitumor 2-(4-aminophenyl)benzothiazoles. J. Med. Chem. 1999, 42, 4172–4184. [Google Scholar] [CrossRef]
- Gong, B.Q.; Hong, F.; Kohm, C.; Bonham, L.; Klein, P. Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. Bioorg. Med. Chem. Lett. 2004, 14, 1455–1459. [Google Scholar] [CrossRef]
- Hutchinson, I.; Bradshaw, T.D.; Matthews, C.S.; Stevens, M.F.G.; Westwell, A.D. Antitumour benzothiazoles. Part 20: 3'-cyano and 3'-alkynyl-substituted 2-(4'-aminophenyl)benzothiazoles as new potent and selective analogues. Bioorg. Med. Chem. Lett. 2003, 13, 471–474. [Google Scholar] [CrossRef]
- Akbay, A.; Ören, I.; Arpaci, Ö.T.; Sener, E.A.; Yalçin, I. Synthesis and HIV-1 Reverse Transcriptase Inhibitor Activity of Some 2,5,6-Substituted Benzoxazole, Benzimidazole, Benzothiazole and Oxazolo(4,5-b)pyridine Derivatives. Arzneimittel-Forsch. 2003, 53, 266–271. [Google Scholar]
- Das, J.; Moquin, R.V.; Lin, J.; Liu, C.J.; Doweyko, A.M.; DeFex, H.F.; Fang, Q.; Pang, S.H.; Pitt, S.; Shen, D.R.; et al. Discovery of 2-amino-heteroarylbenzothiazole-6-anilides as potent p56(lck) inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 2587–2590. [Google Scholar] [CrossRef]
- Hays, S.J.; Rice, M.J.; Ortwine, D.F.; Johnson, G.; Schwarz, R.D.; Boyd, D.K.; Copeland, L.F.; Vartanian, M.G.; Boxer, P.A. Substituted 2-Benzothiazolamine as Sodium Flux Inhibitors—Quantitative Structure-Activity-Relationships and Anticonvulsant Activity. J. Pharm. Sci. 1994, 83, 1425–1432. [Google Scholar] [CrossRef]
- Foscolos, G.; Tsatsas, G.; Champagnac, A.; Pommier, M. Synthesis and pharmacodynamic study of new derivatives of benzothiazole. Ann. Pharm. Fr. 1977, 35, 295–307. [Google Scholar]
- Paget, C.J.; Kisner, K.; Stone, R.L.; DeLong, D.C. Heterocyclic substituted ureas. II. Immunosuppressive and antiviral activity of benzothiazolyl- and benzoxazolylureas. J. Med. Chem. 1969, 12, 1016–1018. [Google Scholar] [CrossRef]
- Sadek, B.; Fahelelbom, K.M.S. Synthesis, Characterization, and Antimicrobial Evaluation of Oxadiazole Congener. Molecules 2011, 16, 4339–4347. [Google Scholar] [CrossRef]
- Türkmen, H.; Ceyhan, N.; Ulkü Karabay Yavaşoğlu, N.; Ozdemir, G.; Cetinkaya, B. Synthesis and antimicrobial activities of hexahydroimidazo[1,5-a]pyridinium bromides with varying benzyl substituents. Eur. J. Med. Chem. 2011, 46, 2895–2900. [Google Scholar] [CrossRef]
- Li, F.; Mulyana, Y.; Feterl, M.; Warner, J.M.; Collins, J.G.; Keene, F.R. The antimicrobial activity of inert oligonuclear polypyridylruthenium(ii) complexes against pathogenic bacteria, including MRSA. Dalton Trans. 2011, 40, 5032–5038. [Google Scholar] [CrossRef]
- Fahelelbom, K.M.S.; Al-Tabakha, M. Quantitative structure activity relationship studies for new antimicrobial N2- substituted phenazines. Afr. J. Pharm. Pharmacol. 2009, 3, 47–50. [Google Scholar]
- Podunavac-Kuzmanović1, S.O.; Cvetković1, D.D.; Barn, D.J. The effect of lipophilicity on the antibacterial activity of some 1-benzylbenzimidazole derivatives. J. Serb. Chem. Soc. 2008, 73, 967–978. [Google Scholar] [CrossRef]
- Vermin, G. General Synthesis Methods for Thiazole and Thiazolium Salts; Metzger, J.V., Ed.; Interscience: New York, NY, USA, 1979; Volume 34, pp. 165–335. [Google Scholar]
- Yoshida, M.; Hayakawa, I.; Hayashi, N.; Agatsuma, T.; Oda, Y.; Tanzawa, F.; Iwasaki, S.; Koyama, K.; Furukawa, H.; Kurakata, S.; Sugano, Y. Synthesis and biological evaluation of benzothiazole derivatives as potent antitumor agents. Bioorg. Med. Chem. Lett. 2005, 15, 3328–3332. [Google Scholar]
- Mcomie, J.F.W.; Watts, M.L.; West, D.E. Demethylation of aryl methyl ethers by boron tribromide. Tetrahedron 1969, 24, 2289–2292. [Google Scholar]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 2007, 12, 139–148. [Google Scholar] [CrossRef]
- Greenberg, M.; Dodds, M.; Tian, M. Naturally occurring phenolic antibacterial compounds show effectiveness against oral bacteria by a quantitative structure-activity relationship study. J. Agric. Food Chem. 2008, 56, 11151–11156. [Google Scholar] [CrossRef]
- Barry, A.L. The Antimicrobial Susceptibility Test: Principle and Practices; Lea and Febiger: Philadelphia, PA, USA, 1976; p. 180, [Biol. Abstr., 64, 25183]. [Google Scholar]
- Sample Availability: Samples of the titled compounds 10–15 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sadek, B.; Al-Tabakha, M.M.; Fahelelbom, K.M.S. Antimicrobial Prospect of Newly Synthesized 1,3-Thiazole Derivatives. Molecules 2011, 16, 9386-9396. https://doi.org/10.3390/molecules16119386
Sadek B, Al-Tabakha MM, Fahelelbom KMS. Antimicrobial Prospect of Newly Synthesized 1,3-Thiazole Derivatives. Molecules. 2011; 16(11):9386-9396. https://doi.org/10.3390/molecules16119386
Chicago/Turabian StyleSadek, Bassem, Moawia Mohammad Al-Tabakha, and Khairi Mustafa Salem Fahelelbom. 2011. "Antimicrobial Prospect of Newly Synthesized 1,3-Thiazole Derivatives" Molecules 16, no. 11: 9386-9396. https://doi.org/10.3390/molecules16119386
APA StyleSadek, B., Al-Tabakha, M. M., & Fahelelbom, K. M. S. (2011). Antimicrobial Prospect of Newly Synthesized 1,3-Thiazole Derivatives. Molecules, 16(11), 9386-9396. https://doi.org/10.3390/molecules16119386