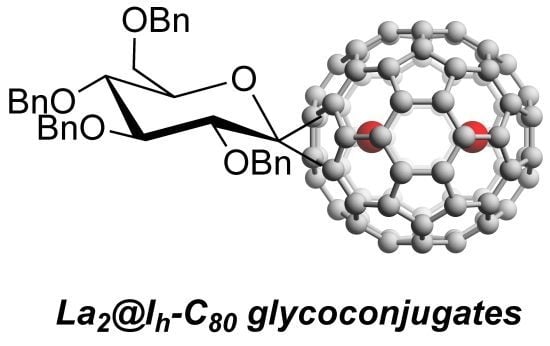
Synthesis of Endohedral Metallofullerene Glycoconjugates by Carbene Addition
Abstract
:1. Introduction
2. Results and Discussion






3. Experimental
3.1. General
3.2. Preparation of La2@Ih-C80 Glycoconjugate (9)
4. Conclusions
Supplementary Materials
Acknowledgements
References and Notes
- Akasaka, T.; Nagase, S. Endofullerenes: A New Family of Carbon Clusters; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef]
- Akasaka, T.; Wudl, F.; Nagase, S. Chemistry of Nanocarbons; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Yamada, M.; Tsuchiya, T.; Akasaka, T.; Nagase, S. In-depth understanding of π-electron systems: New vistas in fullerene endohedrals. Pure Appl. Chem. 2010, 82, 757–767. [Google Scholar] [CrossRef]
- Mikawa, M.; Kato, H.; Okumura, M.; Narazaki, M.; Kanazawa, Y.; Miwa, N.; Shinohara, H. Paramagnetic water soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjugate Chem. 2001, 12, 510–514. [Google Scholar] [CrossRef]
- Bolskar, R.D.; Benedetto, A.F.; Husebo, L.O.; Price, R.E.; Jackson, E.F.; Wallace, S.; Wilson, L.J.; Alford, J.M. First soluble M@C60 derivatives provide enhanced access to metallofullerenes and permit in vivo evaluation of Gd@C60[C(COOH)2]10 as a MRI contrast agent. J. Am. Chem. Soc. 2003, 125, 5471–5478. [Google Scholar]
- Kato, H.; Kanazawa, Y.; Okumura, M.; Taninaka, A.; Yokawa, T.; Shinohara, H. Lanthanoid endohedral metallofullerenols for MRI contrast agents. J. Am. Chem. Soc. 2003, 125, 4391–4397. [Google Scholar] [CrossRef]
- Sitharaman, B.; Bolskar, R.D.; Rusakova, I.; Wilson, L.J. Gd@C60[C(COOH)2]10 and Gd@C60(OH)x: Nanoscale aggregation studies of two metallofullerene MRI contrast agents in aqueous solution. Nano Lett. 2004, 4, 2373–2378. [Google Scholar] [CrossRef]
- Toth, E.; Bolskar, R.D.; Borel, A.; Gonzalez, G.; Helm, L.; Merbach, A.E.; Sitharaman, B.; Wilson, L.J. Water soluble gadofullerenes: Toward high-relaxivity, pH-responsive MRI contrast agents. J. Am. Chem. Soc. 2005, 127, 799–805. [Google Scholar]
- Shu, C.Y.; Zhang, E.Y.; Xiang, J.F.; Zhu, C.F.; Wang, C.R.; Pei, X.L.; Han, H.B. Aggregation studies of the water-soluble gadofullerene magnetic resonance imaging contrast agent: [Gd@C82O6(OH)16(NHCH2CH2COOH)8]x. J. Phys. Chem. B 2006, 110, 15597–15601. [Google Scholar]
- Shu, C.Y.; Gan, L.H.; Wang, C.R.; Pei, X.L.; Han, H.B. Synthesis and characterization of a new water soluble endohedral metallofullerene for MRI contrast agents. Carbon 2006, 44, 496–500. [Google Scholar] [CrossRef]
- Fatouros, P.P.; Corwin, F.D.; Chen, Z.-J.; Broaddus, W.C.; Tatum, J.L.; Kettenmann, B.; Ge, Z.; Gibson, H.W.; Russ, J.L.; Leonard, A.P.; Duchamp, J.C.; Dorn, H.C. In vitro and in vivo imaging studies of a new endohedral metallofullerene nanoparticle. Radiology 2006, 240, 756–764. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Shu, C.Y.; Feng, L.; Wang, C.R. Preparation and characterization of two new water-soluble endohedral metallofullerenes as magnetic resonance imaging contrast agents. J. Phys. Chem. B 2007, 111, 14223–14226. [Google Scholar] [CrossRef]
- MacFarland, D.K.; Walker, K.L.; Lenk, R.P.; Wilson, S.R.; Kumar, K.; Kepley, C.L.; Garbow, J.R. Hydrochalarones: A novel endohedral metallofullerene platform for enhancing magnetic resonance imaging contrast. J. Med. Chem. 2008, 51, 3681–3683. [Google Scholar]
- Shu, C.Y.; Wang, C.R.; Zhang, J.F.; Gibson, H.W.; Dorn, H.C.; Corwin, F.D.; Fatouros, P.P.; Dennis, T.J.S. Organophosphonate functionalized Gd@C82 as a magnetic resonance imaging contrast agent. Chem. Mater. 2008, 20, 2106–2109. [Google Scholar] [CrossRef]
- Shu, C.Y.; Ma, X.Y.; Zhang, J.F.; Corwin, F.D.; Sim, J.H.; Zhang, E.Y.; Dorn, H.C.; Gibson, H.W.; Fatouros, P.P.; Wang, C.R.; Fang, X.H. Conjugation of a water-soluble gadolinium endohedral fulleride with an antibody as a magnetic resonance imaging contrast agent. Bioconjugate Chem. 2008, 19, 651–655. [Google Scholar] [CrossRef]
- Shu, C.Y.; Corwin, F.D.; Zhang, J.F.; Chen, Z.J.; Reid, J.E.; Sun, M.H.; Xu, W.; Sim, J.H.; Wang, C.R.; Fatouros, P.P.; Esker, A.R.; Gibson, H.W.; Dorn, H.C. Facile preparation of a new gadofullerene-based magnetic resonance imaging contrast agent with high 1H relaxivity. Bioconjugate Chem. 2009, 20, 1186–1193. [Google Scholar] [CrossRef]
- Zhang, J.; Fatouros, P.P.; Shu, C.; Reid, J.; Owens, L.S.; Cai, T.; Gibson, H.W.; Long, G.L.; Corwin, F.D.; Chen, Z.-J.; Dorn, H.C. High relaxivity trimetallic nitride (Gd3N) metallofullerene MRI contrast agents with optimized functionality. Bioconjugate Chem. 2010, 21, 610–615. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral metal atoms in pristine and functionalized fullerene cages. Acc. Chem. Res. 2010, 43, 92–102. [Google Scholar] [CrossRef]
- Maeda, Y.; Matsunaga, Y.; Wakahara, T.; Takahashi, S.; Tsuchiya, T.; Ishitsuka, M.O.; Hasegawa, T.; Akasaka, T.; Liu, M.T.H.; Kokura, K.; et al. Isolation and characterization of a carbene derivative of La@C82. J. Am. Chem. Soc. 2004, 126, 6858–6859. [Google Scholar]
- Yamada, M.; Someya, C.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Yoza, K.; Horn, E.; Liu, M.T.H.; Mizorogi, N.; Nagase, S. Metal atoms collinear with the spiro carbon of 6,6-open adducts, M2@C80(Ad) (M = La and Ce, Ad = adamantylidene). J. Am. Chem. Soc. 2008, 130, 1171–1176. [Google Scholar]
- Ishitsuka, M.O.; Enoki, H.; Tsuchiya, T.; Slanina, Z.; Mizorogi, N.; Nagase, S.; Liu, M.T.H.; Akasaka, T. Chemical modification of endohedral metallofullerene La@C82 with 3-chloro-3-phenyldiazirine. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1124–1130. [Google Scholar] [CrossRef]
- Ishitsuka, M.O.; Sano, S.; Enoki, H.; Sato, S.; Nikawa, H.; Tsuchiya, T.; Slanina, Z.; Mizorogi, N.; Liu, M.T.H.; Akasaka, T.; Nagase, S. Regioselective bis-functionalization of endohedral dimetallofullerene, La2@C80: External La-La distance. J. Am. Chem. Soc. 2011, 133, 7128–7134. [Google Scholar]
- Vasella, A.; Uhlmann, P.; Waldraff, C.A.A.; Diederich, F.; Thilgen, C. Fullerene sugars: Preparation of enantiomerically pure, spiro-linked C-glycosides of C60. Angew. Chem. Int. Ed. Engl. 1992, 31, 1388–1390. [Google Scholar] [CrossRef]
- Litvinova, L.S.; Ivanov, V.G.; Mokeev, M.V.; Zgonnik, V.N. Water-soluble [60]fullerene compositions with carbohydrates. Mendeleev Commun. 2001, 11, 193–194. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. Synthesis of [60]fulleropyrrolidine glycoconjugates using 1,3-dipolar cycloaddition with C-glycosyl azomethine ylides. Tetrahedron Lett. 2002, 43, 1649–1652. [Google Scholar] [CrossRef]
- Mikata, Y.; Takagi, S.; Tanahashi, M.; Ishii, S.; Obata, M.; Miyamoto, Y.; Wakita, K.; Nishisaka, T.; Hirano, T.; Ito, T.; Hoshino, M.; Ohtsuki, C.; Tanihara, M.; Yano, S. Detection of 1270 nm emission from singlet oxygen and photocytotoxic property of sugar-pendant [60] fullerenes. Bioorg. Med. Chem. Lett. 2003, 13, 3289–3292. [Google Scholar]
- Enes, R.F.; Tome, A.C.; Cavaleiro, J.A.S.; El-Agamey, A.; McGarvey, D.J. Synthesis and solvent dependence of the photophysical properties of [60]fullerene-sugar conjugates. Tetrahedron 2005, 61, 11873–11881. [Google Scholar] [CrossRef]
- Isobe, H.; Cho, K.; Solin, N.; Werz, D.B.; Seeberger, P.H.; Nakamura, E. Synthesis of fullerene glycoconjugates via a copper-catalyzed Huisgen cycloaddition reaction. Org. Lett. 2007, 9, 4611–4614. [Google Scholar]
- Tanimoto, S.; Sakai, S.; Matsumura, S.; Takahashi, D.; Toshima, K. Target-selective photo-degradation of HIV-1 protease by a fullerene-sugar hybrid. Chem. Commun. 2008, 5767–5769. [Google Scholar]
- Ishida, Y.; Tanimoto, S.; Takahashi, D.; Toshima, K. Photo-degradation of amyloid ® by a designed fullerene–sugar hybrid. Med. Chem. Commun. 2010, 1, 212–215. [Google Scholar] [CrossRef]
- Sanchez-Navarro, M.; Munoz, A.; Illescas, B.M.; Rojo, J.; Martin, N. [60]Fullerene as multivalent scaffold: Efficient molecular recognition of globular glycofullerenes by Concanavalin A. Chem. Eur. J. 2011, 17, 766–799. [Google Scholar]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.-F.; Taganna, J.; Bouckaert, J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH—Towards novel bacterial antiadhesives. Chem. Commun. 2011, 47, 1321–1323. [Google Scholar]
- Beer, D.; Vasella, A. 239. Aldonhydroximo-lactones. Preparation and determination of configuration. Helv. Chim. Acta 1985, 68, 2254–2274. [Google Scholar] [CrossRef]
- Briner, K.; Vasella, A. 153. Glycosylidene carbenes. A new approach to glycoside synthesis. Part 1. Preparation of glycosylidene-derived diaziridines and diazirines. Helv. Chim. Acta 1989, 72, 1371–1382. [Google Scholar] [CrossRef]
- Tate, M.E.; Bishop, C.T. Preparation, thin layer chromatography, and ultraviolet spectra of some O-benzyl derivatives of D-glucos. Can. J. Chem. 1963, 41, 1801–1806. [Google Scholar] [CrossRef]
- Koto, S.; Morishima, N.; Miyata, Y.; Zen, S. Preparation of 2,3,4,6-tetra-O-benzyl-D-mannose. Bull. Chem. Soc. Jpn. 1976, 49, 2639–2640. [Google Scholar] [CrossRef]
- Aebischer, B.M.; Hanssen, H.W.; Vasella, A.T.; Schweizer, W.B. Oxidation of aldose oximes. Formation and structure of hydroxydiazene oxide acetals and preparation of hydroximolactones. X-ray crystal structure of 2,3:5,6-di-O-isopropylidene-α-D-mannofuranosyl-ONN-azoxy 2,3:5,6-di-O-isopropylidene-α-D-mannofuranoside. J. Chem. Soc. Perkin Trans. 1 1982, 2139-2147. [Google Scholar]
- Karplus, M.; Anderson, D.H. Valence-bond interpretation of electron-coupled nuclear spin interactions; application to methane. J. Chem. Phys. 1959, 30, 6–10. [Google Scholar] [CrossRef]
- Karplus, M. Contact electron-spin coupling of nuclear magnetic moments. J. Chem. Phys. 1959, 30, 11–15. [Google Scholar] [CrossRef]
- Karplus, M. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Suzuki, T.; Maruyama, Y.; Kato, T.; Kikuchi, K.; Nakao, Y.; Achiba, Y.; Kobayashi, K.; Nagase, S. Electrochemistry and ab initio study of the dimetallofullerene La2@C80. Angew. Chem. Int. Ed. Engl. 1995, 34, 1094–1096. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamada, M.; Someya, C.I.; Nakahodo, T.; Maeda, Y.; Tsuchiya, T.; Akasaka, T. Synthesis of Endohedral Metallofullerene Glycoconjugates by Carbene Addition. Molecules 2011, 16, 9495-9504. https://doi.org/10.3390/molecules16119495
Yamada M, Someya CI, Nakahodo T, Maeda Y, Tsuchiya T, Akasaka T. Synthesis of Endohedral Metallofullerene Glycoconjugates by Carbene Addition. Molecules. 2011; 16(11):9495-9504. https://doi.org/10.3390/molecules16119495
Chicago/Turabian StyleYamada, Michio, Chika I. Someya, Tsukasa Nakahodo, Yutaka Maeda, Takahiro Tsuchiya, and Takeshi Akasaka. 2011. "Synthesis of Endohedral Metallofullerene Glycoconjugates by Carbene Addition" Molecules 16, no. 11: 9495-9504. https://doi.org/10.3390/molecules16119495
APA StyleYamada, M., Someya, C. I., Nakahodo, T., Maeda, Y., Tsuchiya, T., & Akasaka, T. (2011). Synthesis of Endohedral Metallofullerene Glycoconjugates by Carbene Addition. Molecules, 16(11), 9495-9504. https://doi.org/10.3390/molecules16119495