Polycationic Glycosides
Abstract
:1. Introduction
2. Results and Discussion

| Product Number | Product Structure | Precursor Tertiary Amine | Yield (%) |
|---|---|---|---|
| 1 |  |  | 94 |
| 2 |  |  | 95 |
| 3 |  |  | 98 |
| 4 |  |  | 99 |
| 5 |  |  | 99 |
| 6 |  |  | 99 |
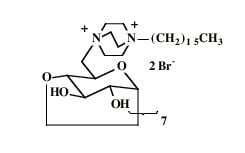
| 7 |  |  | 99 |
| 8 |  |  | 91 |
| 9 |  |  | 99 |
| 10 |  |  | 33 |
| 11 |  |  | 29 |
| 12 |  |  | 33 |
| 13 |  |  | 31 |
| 14 |  |  | 30 |
| 15 |  |  | 42 |
| 16 |  |  | 56 |
| Product Number | 1H-NMR (D2O)(δ) | 13C-NMR (δ) | Elemental Analysis |
|---|---|---|---|
| 1 | 1.36 (6H) d, 2.31 (3H) s, 3.28-3.80 (20H) br, 7.28-7.62 (4H) AA'BB' | 15.5, 20.5, 44.0, 47.8, 55.1, 60.6, 68.3, 69.6, 71.2, 71.6, 73.1, 99.3, 125.4, 129.5, 139.5, 142.5 | Calcd. for C23H39BrN2O8S.8.5H2O: C, 37.50; H, 7.66. Found: C 37.53; H, 7.37 |
| 2 | 1.01 (6H) d, 2.21 (1H) br, 2.30 (3H) s, 3.35 (3H) s, 3.33-3.87 (21H) m, 7.27-7.60 (4H) AA'BB' | 20.6, 21.9, 22.9, 44.0, 51.2, 55.1, 60.6, 69.6, 71.3, 71.6, 73.1, 74.2, 99.3, 125.4, 129.6, 139.6, 142.5 | Calcd. for C24H41BrN2O8S.9H2O: C, 37.94; H, 7.83. Found: C 8.12; H, 7.79 |
| 3 | 0.83 (3H) t, 1.28 (2H) m, 1.64 (2H) m, 2.29 (3H) s, 3.29-3.50 (5H) br, 3.69-3.80 (16H) br, 3.81 (3H) br, 7.26-7.58 (4H) AA'BB' | 12.7, 18.9, 20.5, 43.9, 46.2, 50.7, 55.0, 60.5, 65.1, 69.5, 71.2, 71.5, 73.0, 99.2, 125.3, 129.5, 139.4, 142.5 | Calcd. for C24H41BrN2O8S.5H2O: C, 41.92; H, 7.48. Found: C 41.65; H, 7.76 |
| 4 | 2.34 (3H) s, 3.36 (3H) s, 3.32-3.84 (19H) br, 4.66 (2H) br, 7.32-7.64 (9H) br | 20.5, 44.1, 50.6, 55.0, 60.6, 69.0, 69.6, 71.3, 71.6, 73.1, 99.3, 125.4, 126.3, 129.5, 129.6, 131.4, 133.0, 139.5, 145.5 | Calcd. for C27H39ClN2O8S.4H2O: C, 50.54; H, 6.99. Found: C 50.51; H, 7.00 |
| 5 | 0.76 (3H) t, 1.17-1.24 (10H) br, 1.69 (2H) br, 2.29 (3H) s, 3.36 (3H) s, 3.30-3.78 (21H) br, 7.26-7.58 (4H) AA'BB' | 13.3, 20.4, 21.3, 21.9, 25.2, 28.0, 28.1, 30.9, 43.9, 50.7, 55.0, 58.1, 60.5, 65.3, 71.2, 71.5, 73.0, 99.2, 125.3, 129.4, 139.4, 142.5 | Calcd. for C28H49BrN2O8S.2H2O: C, 48.76; H, 7.74. Found: C 48.77; H, 7.80 |
| 6 | 0.84 (3H) t, 0.86-1.01 (18H) br, 1.24 (2H) br, 2.19 (3H) s, 3.32 (3H) br, 3.43-3.76 (21H) br, 7.08-7.57 (4H) AA'BB' | 13.7, 20.8, 21.2, 22.5, 23.8, 28.2, 29.3, 29.4, 29.6, 29.7, 30.2, 31.8, 43.9, 46.9, 50.9, 55.0, 60.5, 69.5, 71.2, 71.2, 73.0, 99.2, 125.6, 129.1, 139.8, 141.2 | Calcd. for C32H57BrN2O8S.2H2O: C, 51.53; H, 8.24. Found: C 51.46; H, 8.31 |
| 7 | 0.84 (3H) t, 0.86-1.01 (22H) br, 1.24 (2H) br, 2.19 (3H) s, 3.32 (3H) s, 3.43-3.76 (21H) br, 7.04-7.58 (4H) AA'BB' | 13.9, 21.0, 21.7, 21.9, 22.8, 26.0, 26.3, 29.1, 29.7, 29.8, 30.0, 30.1, 32.2, 44.1, 46.4, 50.8, 55.1, 60.6, 64.7, 69.6, 71.3, 71.6, 73.2, 99.3, 125.8, 129.1, 139.8, 142.1 | Calcd. for C34H61BrN2O8S.2H2O: C, 52.77; H, 8.47. Found: C 52.83; H, 8.52 |
| 8 | 0.81 (3H) t, 1.19 (26H) br, 1.69 (2H) br, 2.15 (3H) s, 3.32 (3H) s, 3.44-3.87 (21H) br, 6.94-7.55 (4H) AA'BB' | 14.1, 21.2, 22.7, 24.5, 26.1, 26.9, 27.1, 28.9, 29.2, 29.5, 29.66, 29.68, 29.73, 29.8, 29.9, 32.7, 44.2, 45.2, 50.6, 55.3, 60.8, 69.7, 71.6, 71.7, 73.4, 99.3, 125.8, 129.1, 140.1, 141.9 | Calcd. for C36H65ClN2O8S.5H2O: C, 46.56; H, 9.55. Found: C 46.53; H, 9.18 |
| 9 | 0.86 (3H) t, 1.24 (30H) br, 1.69 (2H) br, 2.29 (3H) s, 3.04 (2H) br, 3.31 (3H) s, 3.19-3.85 (19H) br, 7.11-7.47 (4H) AA'BB' | 13.9, 21.2, 22.0, 22.8, 24.3, 26.2, 26.9, 28.9, 29.44, 29.47, 29.50, 29.6, 29.67, 29.70, 29.75, 29.8, 29.9, 32.7, 44.6, 45.7, 50.9, 55.4, 61.0, 69.9, 71.6, 71.8, 73.7, 99.6, 125.4, 128.0, 141.0, 142.1 | Calcd. for C38H69BrN2O8S5H2O: C, 51.63; H, 9.01. Found: C 51.55; H, 9.23 |
| 11 | 0.86 (18H) t, 1.26 (156H) br, 1.64 (12H) br, 3.35 (12H) br, 3.55-3.90 (108H) br, 5.03 (6H) d | 15.33, 23.41, 24.28, 24.31, 24.35, 27.48, 28.22, 28.71, 30.45, 30.68, 30.99, 31.41, 31.87, 31.93, 33.80, 45.99, 52.88, 60.97, 66.03, 72.19, 73.91, 75.76, 83.04, 103.94 | Calcd. for C168H324Br12N12O244H2O: C, 51.38; H, 8.52. Found: C 51.29; H, 8.63 |
| 12 | 0.85 (21H) t, 1.16 (126H) br, 1.60 (14H) br, 3.25 (14H) br, 3.48-3.78 (126H) br, 4.96 (7H) d | 13.74, 21.02, 21.72, 22.34, 25.82, 28.64, 28.76, 28.87, 28.92, 29.04, 31.48, 44.20, 51.20, 51.86, 60.02, 65.25, 72.17, 73.61, 81.26, 102.38 | Calcd. for C168H322Br14N14O283H2O: C, 48.51; H, 7.95. Found: C 48.36; H, 8.18 |
| 13 | 0.86 (21H) t, 1.27 (182H) br, 1.65 (14H) br, 3.36 (14H) br, 3.45-3.81 (126H) br, 4.99 (7H) d | 15.37, 23.42, 24.22, 24.35, 24.39, 27.44, 28.20, 28.75, 30.41, 30.57, 30.97, 31.28, 31.91, 31.94, 33.79, 46.01, 52.85, 60.86, 66.11, 72.23, 73.87, 75.82, 83.10, 103.96 | Calcd. for C196H378Br14N14O283H2O: C, 51.72; H, 8.50. Found: C 51.66; H, 8.58 |
| 14 | 0.86 (21H) t, 1.28 (266H) br, 1.66 (14H) br, 3.37 (14H) br, 3.44-3.80 (126H) br, 4.99 (7H) d | 15.34, 23.41, 24.29, 24.37, 24.42, 24.58, 25.36, 27.50, 28.19, 28.83, 29.24, 30.43, 30.54, 30.61, 30.95, 31.30, 31.67, 31.93, 31.95, 33.72, 33.81, 46.03, 52.91, 60.92, 66.18, 72.27, 73. 95, 75.80, 83.17, 103.91 | Calcd. for C238H462Br14N14O283H2O: C, 55.60; H, 9.18. Found: C 55.53; H, 9.22 |
| 15 | 0.79-0.85 (9H) br, 1.23 (54H) br, 1.71 (6H) br, 2.14 (9H) s, 3.40-3.88 (56H) br, 6.93-7.53 (12H) AA'BB' | 13.71, 14.22, 14.76, 19.73, 20.28, 20.35, 22.57, 22.86, 23.31, 23.79, 24.00, 24.43, 24.78, 25.25, 25.63, 26.04, 26.41, 26.80, 27.07, 27.65, 27.97, 28.36, 28.72, 29.23, 29.79, 30.30, 30.56, 31.18, 31.66, 32.18, 32.67, 32.94, 33.34, 44.89, 42.62, 43.01, 43.17, 44.55, 45.31, 45.87, 46.18, 46.32, 47.72, 47.93, 48.29, 49.50, 52.22, 59.48, 60.31, 65.64, 71.75, 73.47, 83.12, 99.93, 103.38, 125.56, 128.84, 141.10, 141.96 | Calcd. for C87H151Cl3N6O17S3H2O: C, 58.91; H, 8.69. Found: C 58.78; H, 8.74 |
| 16 | 0.80-0.85 (9H) br, 1.21 (78H) br, 1.70 (6H) br, 2.15 (9H) s, 3.42-3.91 (56H) br, 6.95-7.55 (12H) AA'BB' | 13.80, 14.13, 14.62, 19.98, 20.11, 20.31, 20.87, 22.43, 22.75, 23.29, 23.44, 23.57, 23.82, 24.10, 24.22, 24.56, 24.90, 25.23, 25.57, 25.87, 26.02, 26.39, 26.75, 27.12, 27.27, 27.54, 27.80, 28.04, 28.23, 28.58, 28.65, 29.18, 29.70, 30.44, 30.67, 31.32, 31.65, 31.99, 32.27, 32.31, 32.54, 32.86, 33.29, 44.65, 42.71, 43.09, 43.18, 44.67, 45.27, 45.73, 46.03, 46.38, 47.52, 47.99, 48.18, 49.82, 52.34, 59.67, 60.21, 65.48, 71.47, 73.76, 82.66, 99.71, 102.84, 125.73, 129.29, 140.02, 141.80 | Calcd. for C99H175Cl3N6O17S3H2O: C, 61.23; H, 9.19. Found: C 61.17; H, 9.28 |
| Compound | S. aureus | P. aeruginosa | A. niger | C. albicans |
|---|---|---|---|---|
| 1 | >5.8 | - | - | - |
| 2 | >5.9 | - | - | - |
| 3 | >5.9 | - | - | - |
| 4 | 3.11 | - | - | - |
| 5 | >6.5 | - | - | - |
| 6 | >6.6 | 12.5 | - | - |
| 7 | 1.99 × 10−1 | - | - | - |
| 8 | >7.2 | - | 4.9 × 10−2 | 2.4 × 10−2 |
| 9 | >7.9 | - | - | - |
| 10 | 4.6 × 10−2 | - | - | - |
| 11 | 2.3 × 10−2 | - | - | - |
| 12 | 2.3 × 10−2 | 3.9 × 10−1 | 2.0 × 10−1 | 4.9 × 10−2 |
| 13 | 2.3 × 10−2 | 1.56 | 4.9 × 10−2 | 2.4 × 10−2 |
| 14 | 2.4 × 10−2 | - | - | - |
| 15 | 1.3 | - | - | - |
| 16 | 2.4 | - | - | - |
| modified soluble starch | 1.3 × 10−7 | - | - | - |
3. Experimental
3.1. General
3.2. Syntheses of cationic lipid appended glycosides
3.3. Evaluation of antimicrobial activity of salts
4. Conclusions
Acknowledgements
References and Notes
- Abel, T.; Cohen, J.I.; Engel, R.; Filshtinskaya, M.; Melkonian, A.; Melkonian, K. Preparation and investigation of carbohydrate-based surfaces. Carbohydr. Res. 2002, 337, 2495–2499. [Google Scholar] [CrossRef]
- Abel, T.; Cohen, J.I.; Escalera, J.; Engel, R.; Filshtinskaya, M.; Fincher, R.; Melkonian, A.; Melkonian, K. Polycations. 14. Preparation and investigation of protein-based antibacterial surfaces. J. Textile Apparel Technol. Manage. 2004, 3, 1–8. [Google Scholar]
- Cohen, J.I.; Abel, T.; Burkett, D.; Engel, R.; Escalera, J.; Filshtinskaya, M.; Hatchett, R.; Leto, M.; Melgar, Y.; Melkonian, K. Polycations. 15. Polyammonium surfaces - a new approach to antifungal activity. Lett. Drug Des. Disc. 2004, 1, 1–3. [Google Scholar]
- Engel, R.; Cohen, J.I.; Melkonian, K. Antimoicrobial surfaces. U.S. Patent 7,285,286, 10 July 2007. [Google Scholar]
- Engel, R.; Cohen, J.I.; Melkonian, K. Antimoicrobial surfaces. U.S. Patent 7,241,453, 23 October 2007. [Google Scholar]
- Engel, R.; Rizzo, J.I.; Rivera, C.; Ramirez, M.; Huang, M.L.; Weiss, H.; Adelkader, O.; Capodiferro, C.; Behaj, V.; Thomas, M.; Engel, J.F. Polycations. 18. The synthesis of polycationic lipid materials based on the diamine 1,4-diazabicyclo[2.2.2]octane. Chem. Phys. Lipids 2009, 158, 61–69. [Google Scholar] [CrossRef]
- Cohen, J.I.; Castro, S.; Han, J.-A.; Shteto, V.; Engel, R. Polycations. IX. Polyammonium derivatives of cyclodextrins - synthesis and binding to organic oxyanions. Heteroat. Chem. 2000, 11, 546–555. [Google Scholar] [CrossRef]
- Fabian, J.; October, T.; Cherestes, A.; Engel, R. Polycations: Syntheses of polyammonium strings as antibacterial agents. Synlett 1997, 1007–1009. [Google Scholar]
- Strekas, T.; Engel, R.; Locknauth, K.; Cohen, J.; Fabian, J. Polycations. 5. Inducement of Ѱ-DNA circular dichroism signals for duplex deoxyribonucleotide homopolymers by polycationic strings. Arch. Biochem. Biophys. 1999, 364, 129–131. [Google Scholar] [CrossRef]
- Cohen, J.I.; Traficante, L.; Schwartz, P.W.; Engel, R. Polycations. 4. Synthesis and antihydrophobic effect of polycationic strings. Tetrahedron Lett. 1998, 39, 8617–8620. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the corresponding author.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Engel, R.; Ghani, I.; Montenegro, D.; Thomas, M.; Klaritch-Vrana, B.; Castaño, A.; Friedman, L.; Leb, J.; Rothman, L.; Lee, H.; et al. Polycationic Glycosides. Molecules 2011, 16, 1508-1518. https://doi.org/10.3390/molecules16021508
Engel R, Ghani I, Montenegro D, Thomas M, Klaritch-Vrana B, Castaño A, Friedman L, Leb J, Rothman L, Lee H, et al. Polycationic Glycosides. Molecules. 2011; 16(2):1508-1518. https://doi.org/10.3390/molecules16021508
Chicago/Turabian StyleEngel, Robert, Ishrat Ghani, Diego Montenegro, Marie Thomas, Barbara Klaritch-Vrana, Alejandra Castaño, Laura Friedman, Jay Leb, Leah Rothman, Heidi Lee, and et al. 2011. "Polycationic Glycosides" Molecules 16, no. 2: 1508-1518. https://doi.org/10.3390/molecules16021508
APA StyleEngel, R., Ghani, I., Montenegro, D., Thomas, M., Klaritch-Vrana, B., Castaño, A., Friedman, L., Leb, J., Rothman, L., Lee, H., Capodiferro, C., Ambinder, D., Cere, E., Awad, C., Sheikh, F., Rizzo, J., Nisbett, L. -M., Testani, E., & Melkonian, K. (2011). Polycationic Glycosides. Molecules, 16(2), 1508-1518. https://doi.org/10.3390/molecules16021508