Synthesis, Characterization and Biological Evaluation of Succinate Prodrugs of Curcuminoids for Colon Cancer Treatment
Abstract
:1. Introduction
2. Results and Discussion
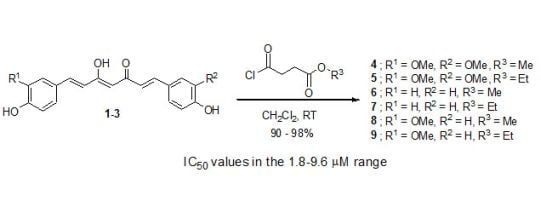
2.1. Synthesis of Curcuminoids 1-3 and Succinyl Derivatives 4-9
2.2. Cytotoxicity Evaluation
2.3. Chemical Stability Study
2.4. Release Study
3. Experimental
3.1. General
3.2. General Method for the Synthesis of Curcumin (1) and Bisdesmethoxycurcumin (2)
3.3. Synthesis of Desmethoxycurcumin (3).
3.4. General Method for Synthesis of Curcuminoid Succinate Ester Derivatives 4–9
3.5. Cytotoxicity Evaluation
3.6. Chemical Stability Study
3.7. Release Study
4. Conclusions
Acknowledgements
References
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Buadonpri, W.; Wichitnithad, W.; Rojsitthisak, P.; Towiwat, P. Synthetic curcumin inhibits carrageenan-induced paw edema in rats. J. Health Res. 2009, 23, 11–16. [Google Scholar]
- Abe, Y.; Hashimoto, S.; Horie, T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lantz, R.C.; Chen, G.J.; Solyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine 2005, 1, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M. Inhibition of tumor necrosis factor by curcumin. Biochem. Pharmacol. 1995, 49, 1551–1556. [Google Scholar] [CrossRef]
- Mishra, S.; Narain, U.; Mishra, R.; Misra, K. Design, development and synthesis of mixed bioconjugates of piperic acid-glycine, curcumin-glycine/alanine and curcumin-glycine-piperic acid and their antibacterial and antifungal properties. Bioorg. Med. Chem. 2005, 13, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.C.; Vatsala, P.G.; Keshamoumi, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Sharma, A.K.; Narain, U.; Misra, K.; Pati, U. Design, synthesis and characterization of some bioactive conjugates of curcumin with glycine, glutamic acid, valine and demethylenated piperic acid and study of their antimicrobial and antiproliferative properties. Eur. J. Med. Chem. 2007, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Raghavan, K.; Weinstein, J.; Kohn, K.W.; Pommier, Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995, 49, 1165–1170. [Google Scholar] [CrossRef]
- Ahmad-Raus, R.R.; Abdul-Latif, E.S.; Mohammad, J.J. Lowering of lipid composition in aorta of guinea pigs by Curcuma domestica. BMC Complement. Altern. Med. 2001, 6, 345–348. [Google Scholar] [CrossRef]
- Eigner, D.; Ferula, S.D. asa-foetida and curcuma longa in traditional medical treatment and diet in Nepal. J. Ethnopharmacol. 1999, 67, 1–6. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Sharma, R.A.; Ireson, C.R.; Verchoyle, R.D.; Hill, K.A.; Williams, M.L.; Leuratti, C.; Manson, M.M.; Marnett, L.J.; Steward, W.P.; Gescher, A. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin. Cancer Res. 2001, 7, 1452–1458. [Google Scholar] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; Yu, H.S.; Jee, S.H.; Chen, G.S.; Chen, T.M.; Chen, C.A.; Lai, M.K.; Pu, Y.S.; Pan, M.H.; Wang, Y.J.; Tsai, C.C.; Hsieh, C.Y. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Hidaka, K,; Shinohara, A.; Maekawa, T,; Takeda, Y.; Yamaguchi, H. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J. Agric. Food Chem. 1999, 47, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narain, U.; Tripathi, S.; Misra, K. Syntheses of curcumin bioconjugates and study of their antibacterial activities against β-lactamase-producing microorganisms. Bioconjugate Chem. 2001, 12, 464–469. [Google Scholar] [CrossRef]
- Ferrari, E.; Lazzari, S.; Marverti, G.; Pignedoli, F.; Spagnolo, F.; Saladini, M. Synthesis, cytotoxic and combined cDDP activity of new stable curcumin derivatives. Bioorg. Med. Chem. 2009, 17, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D.; Taphouse, V. Initial Rate Studies of Hydrolysis and Acyl Migration in Methylprednisolone 21-Hemisuccinate and 17-Hemisuccinate. J. Pharm. Sci. 1981, 70, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Garrett, E.R. Prediction of Stability in Pharmaceutical Preparations X. Alkaline Hydrolysis of Hydrocortisone Hemisuccinate. J. Pharm Sci. 1962, 51, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Brent, D.A.; Chandrasurin, P.; Ragouzeos, A.; Hurlbert, B.S.; Burke, J.T. Rearrangement of Chloramphenicol-3-monosuccinate. J. Pharm. Sci. 1980, 69, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.; Larsen, C. Stability and Kinetics of Hydrolysis of Metronidazole Monosuccinate in Aqueous Solution and in Plasma. Int. J. Pharm. 1984, 21, 201–209. [Google Scholar] [CrossRef]
- La-Scalea, M.A.; Menezes, C.M.S.; Masutami, G.C.; Polli, M.C; Serrano, S.H.P.; Ferreira, E.I. Molecular modeling of the voltammetric oxidation at a glassy carbon electrode of the antimalarial drug primaquine and its prodrugs succinylprimaquine and maleylprimaquine. Electrochim. Acta 2006, 51, 5103–5111. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Ramachandra, M.S.; Subbaraju, G.V. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg. Med. Chem. 2005, 13, 6437–6380. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, desmethoxycurcumin, and bisdesmethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Shi, Q.; Nyarko, A.K.; Bastow, K.F.; Wu, C.C.; Su, C.Y.; Shih, C.C.; Lee, K.H. Antitumor agents. 250. Design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. J. Med. Chem. 2006, 49, 3963–3972. [Google Scholar] [CrossRef] [PubMed]
- Wichitnithad, W.; Jongaroonngamsang, N.; Pummangura, S.; Rojsitthisak, P. A simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts. Phytochem. Anal. 2009, 20, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Sreenivasan, K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J. Pharm Sci. 2011, 100, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Safavy, A.; Raisch, K.P.; Mantena, S.; Sanford, L.L.; Sham, S.W.; Krishna, N.R.; Bonner, J.A. Design and development of water-soluble curcumin conjugates as potential anticancer agents. J. Med. Chem. 2007, 50, 6284–6288. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Milroy, R.; Kaye, S.B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989, 49, 4435–4440. [Google Scholar] [PubMed]
Sample Availability: Samples of compounds 1–9 are available from the authors. |







| Compounds | IC50 (μM) ± SD |
|---|---|
| 1 | 3.31 ± 0.16 |
| 2 | 4.93 ± 0.21 |
| 3 | 3.36 ± 0.26 |
| 4 | 3.84 ± 0.19 |
| 5 | 1.84 ± 0.11 |
| 6 | 3.78 ± 0.31 |
| 7 | 5.97 ± 0.28 |
| 8 | 4.40 ± 0.15 |
| 9 | 9.60 ± 0.31 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, Characterization and Biological Evaluation of Succinate Prodrugs of Curcuminoids for Colon Cancer Treatment. Molecules 2011, 16, 1888-1900. https://doi.org/10.3390/molecules16021888
Wichitnithad W, Nimmannit U, Wacharasindhu S, Rojsitthisak P. Synthesis, Characterization and Biological Evaluation of Succinate Prodrugs of Curcuminoids for Colon Cancer Treatment. Molecules. 2011; 16(2):1888-1900. https://doi.org/10.3390/molecules16021888
Chicago/Turabian StyleWichitnithad, Wisut, Ubonthip Nimmannit, Sumrit Wacharasindhu, and Pornchai Rojsitthisak. 2011. "Synthesis, Characterization and Biological Evaluation of Succinate Prodrugs of Curcuminoids for Colon Cancer Treatment" Molecules 16, no. 2: 1888-1900. https://doi.org/10.3390/molecules16021888
APA StyleWichitnithad, W., Nimmannit, U., Wacharasindhu, S., & Rojsitthisak, P. (2011). Synthesis, Characterization and Biological Evaluation of Succinate Prodrugs of Curcuminoids for Colon Cancer Treatment. Molecules, 16(2), 1888-1900. https://doi.org/10.3390/molecules16021888