Effects of Agronomic Practices on Volatile Composition of Hyssopus officinalis L. Essential Oils
Abstract
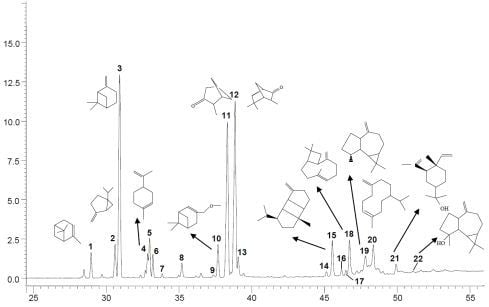
:1. Introduction
2. Results and Discussion

| Batch from non-irrigated crop (%) | Batch from irrigated crop (%) | ISO 9841:2007 (E) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| Average Oil volume (L/Batch) | 5.25 | 5.25 | 6.50 | 6.63 | 6.67 | 6.75 | 7.00 | 6.20 | 6.25 | 6.25 | |||
| EO yield extraction (%) | 0.35a | 0.35a | 0.43b | 0.44b | 0.44b | 0.45b | 0.47c | 0.41b | 0.42b | 0.42b | |||
| Compounds identification | KI | % | |||||||||||
| 1 | α -pinene | 939 | 1.87e | 1.83de | 1.84e | 1.77cd | 1.75c | 1.60ab | 1.54a | 1.58ab | 1.62b | 1.61ab | 0.4 - 1.5 |
| 2 | Sabinene | 975 | 2.85b | 2.75ab | 2.74ab | 2.73ab | 2.70a | 2.65a | 2.68a | 2.64a | 2.72a | 2.62a | 1.0 - 3.5 |
| 3 | β -pinene | 979 | 20.33cd | 19.88cd | 20.54d | 19.67cd | 19.25bc | 18.33ab | 17.72a | 17.62a | 17.73a | 17.65a | 7.0 - 20 |
| 4 | Limonene | 1029 | 1.73c | 1.61b | 1.53a | 1.57ab | 1.58ab | 2.09e | 2.01e | 1.93d | 2.03e | 2.22f | 0.6 - 4.0 |
| 11 | Pinocamphone | 1162 | 17.16a | 17.06a | 16.87a | 17.38ab | 17.62ab | 18.53c | 17.07a | 17.94bc | 19.33d | 19.75d | 8.0 - 25 |
| 12 | iso -pinocamphone | 1175 | 19.79a | 19.36a | 19.47a | 19.97a | 20.62ab | 21.43b | 24.58c | 24.22c | 23.65c | 23.93c | 25 - 45 |
| 10 | myrtenyl methyl ether | 1372 | 2.86a | 2.81a | 3.01b | 3.07b | 3.13b | 3.15b | 3.31c | 3.33c | 3.33c | 3.36c | 0.9 - 3.0 |
| 15 | β -bourbonene | 1388 | 3.48a | 3.91b | 4.07bc | 4.08bc | 4.32c | 4.11bc | 3.91b | 4.05bc | 4.19c | 4.25c | 0.8 - 2.6 |
| 18 | β -caryophyllene | 1419 | 3.88c | 4.16d | 4.16d | 3.94cd | 3.96cd | 3.56b | 3.11a | 3.29a | 3.27a | 3.28a | 1.0 - 3.0 |
| 19 | allo -aromadendrene | 1460 | 2.62a | 3.16a | 3.07a | 2.98a | 2.94a | 3.03a | 3.12a | 2.77a | 2.58a | 2.56a | 1.0 - 3.0 |
| 20 | germacrene-D | 1481 | 4.76f | 4.43ef | 4.28e | 4.04de | 3.63bcd | 3.70cd | 3.38abc | 3.38abc | 3.23ab | 3.06a | 1.2 - 4.5 |
| 21 | Elemol | 1549 | 0.97a | 1.19bc | 1.29c | 1.19bc | 1.23c | 0.96a | 1.02ab | 0.96a | 0.91a | 0.83a | 0.2 -2.5 |
| 22 | Spathulenol | 1578 | 0.14a | 0.25ab | 0.27ab | 0.42b | 0.31ab | 0.37b | 0.30ab | 0.32ab | 0.34ab | 0.29ab | 0.1 -1.5 |
| Percentage for ISO compounds | 82.44 | 82.40 | 83.14 | 82.81 | 83.05 | 83.49 | 83.78 | 84.04 | 84.94 | 85.42 | |||
| 5 | β -phellandrene | 1025 | 3.14e | 3.02de | 2.96cd | 2.89bcd | 2.87abc | 2.89bcd | 3.92g | 3.44f | 2.80ab | 2.74a | N.I |
| 6 | (E)- β -ocimene | 1050 | 1.57f | 1.46e | 1.32d | 1.31d | 1.18c | 1.12b | 1.15bc | 1.09b | 1.09ab | 1.04a | N.I |
| 7 | γ -terpinene | 1059 | 0.31a | 0.28a | 0.27a | 0.27a | 0.27a | 0.29a | 0.29a | 0.29a | 0.25a | 0.40a | N.I |
| 8 | Linalool | 1096 | 1.30a | 1.23a | 1.25a | 1.23a | 1.28a | 1.23a | 1.23a | 1.13a | 1.21a | 1.14a | N.I |
| 9 | Pinocarveol | 1184 | 0.25a | 0.25a | 0.30b | 0.30b | 0.34c | 0.41d | 0.42d | 0.46e | 0.48f | 0.50f | N.I |
| 13 | Myrtenol | 1195 | 1.82c | 1.77bc | 1.72bc | 1.78c | 1.75bc | 1.81c | 0.69a | 1.07ab | 1.07ab | 1.00a | N.I |
| 14 | methyl eugenol | 1402 | 0.43a | 0.48a | 0.45a | 0.44a | 0.49a | 0.47a | 0.44a | 0.46a | 0.48a | 0.48a | N.I |
| 16 | α -gurjunene | 1409 | 0.54ab | 0.60bc | 0.60bc | 0.60bc | 0.61c | 0.58abc | 0.55abc | 0.53ab | 0.48a | 0.48a | N.I |
| 17 | Bicyclogermacrene | 1500 | 0.50a | 0.56b | 0.56b | 0.57b | 0.57b | 0.53ab | 0.49a | 0.52ab | 0.53ab | 0.53ab | N.I |
| Percentage for other compounds (no ISO) | 9.87 | 9.63 | 9.44 | 9.39 | 9.35 | 9.33 | 9.18 | 9.00 | 8.38 | 8.30 | |||
| Percentage of total area compounds | 92.31 | 92.04 | 92.57 | 92.20 | 92.40 | 92.82 | 92.96 | 93.03 | 93.32 | 93.72 | |||

3. Experimental
3.1. Plant Material
3.2. Essential Oil Extraction
3.3. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
3.4. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Kerrola, K.; Galambosi, B.; Kallio, H. Volatile components and odor intensity of four phenotypes of Hyssop (Hyssopus officinalis L.). J. Agr. Food Chem. 1994, 42, 776–781. [Google Scholar]
- Fraternale, D.; Ricci, D. Composition and antifungal activity of two essential oils of Hyssop (Hyssopus officinalis L.). J. Essent. Oil Res. 2004, 16, 617–622. [Google Scholar] [CrossRef]
- Höld, K.M.; Sirisoma, N.S.; Sparks, S.E; Casida, J.E. Metabolism and mode of actions of cis- and trans-3-pinanones the active ingredients of hyssop oil). Xenobiotica 2002, 32, 251–265. [Google Scholar] [CrossRef]
- Özer, H.; Sahin, F.; Kiliç, H.; Güllüce, M. Essential Oil composition of Hyssopus officinalis L. subsp. angustifolius (Bieb.) Arcangeli from Turkey. Flav. Frag. J. 2005, 20, 42–44. [Google Scholar] [CrossRef]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar]
- The European Enviroment Agency (EEA) Web site. Available online: http://eunis.eea.europa.eu accessed on 13 October 2010.
- Garg, S.N.; Naqvi, A.A.; Singh, A.; Ram, G.; Kumar, S. Composition of essential oil from an annual crop of Hyssopus officinalis grown in Indian plains. Flav. Frag. J. 1999, 14, 170–172. [Google Scholar] [CrossRef]
- Mazzanti, G.; Battinelli, L.; Salvatore, G. Antimicrobial properties of the linalool-rich essential oil of Hyssopus officinalis L. var decumbens (Lamiaceae). Flav. Frag. J. 1998, 13, 289–294. [Google Scholar] [CrossRef]
- Rohloff, J.; Dragland, S.; Mordal, R.; Iversen, T. Effect of harvest time and drying method on biomass production, essential oil yield, and quality of Peppermint (Mentha × piperita L.). J. Agr. Food Chem. 2005, 53, 4143–4148. [Google Scholar]
- ISO, Oil of Hyssop Hyssopus officinalis L. ssp. officinalis, 2nd ed; International Organisation for Standardization: Geneva, Switzerland, 2007.
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Sci. Hort. 2008, 115, 393–397. [Google Scholar] [CrossRef]
- The Meteorological National Agency of Spain (AEMET) Website. Available online: http://www.aemet.es/es/elclima/datosclimatologicos/valoresclimatologicos?k=clm accessed on 12 November 2010.
- Millet, Y.; Jouglard, J.; Steinmetz, M.D. Toxicity of some essentials plants oil. Clinical and experimental study. Clin. Toxicol. 1981, 18, 1485–1498. [Google Scholar] [CrossRef]
- Belletti, N.; Kamdem, S.S.; Tabanelli, G.; Lanciotti, R.; Gardini, F. Modeling of combined effects of citral, linalool and β-pinene used against Saccharomyces cerevisiae in citrus-based beverages subjected to a mild heat treatment. Int. J. Food Microbiol. 2010, 136, 283–289. [Google Scholar]
- Belletti, N.; Ndagijimana, M.; Sisto, C.; Guerzoni, M.E.; Lanciotti, R.; Gardini, F. Evaluation of the antimicrobial activity of citrus essences on Saccharomyces cerevisiae. J. Agr. Food Chem. 2004, 52, 6932–6938. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectometry; Kozlowski, A.C., Ed.; Allured Business Media: Carol Stream, IL, USA, 2009. [Google Scholar]
- Samples Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moro, A.; Zalacain, A.; Hurtado de Mendoza, J.; Carmona, M. Effects of Agronomic Practices on Volatile Composition of Hyssopus officinalis L. Essential Oils. Molecules 2011, 16, 4131-4139. https://doi.org/10.3390/molecules16054131
Moro A, Zalacain A, Hurtado de Mendoza J, Carmona M. Effects of Agronomic Practices on Volatile Composition of Hyssopus officinalis L. Essential Oils. Molecules. 2011; 16(5):4131-4139. https://doi.org/10.3390/molecules16054131
Chicago/Turabian StyleMoro, Armando, Amaya Zalacain, Jorge Hurtado de Mendoza, and Manuel Carmona. 2011. "Effects of Agronomic Practices on Volatile Composition of Hyssopus officinalis L. Essential Oils" Molecules 16, no. 5: 4131-4139. https://doi.org/10.3390/molecules16054131
APA StyleMoro, A., Zalacain, A., Hurtado de Mendoza, J., & Carmona, M. (2011). Effects of Agronomic Practices on Volatile Composition of Hyssopus officinalis L. Essential Oils. Molecules, 16(5), 4131-4139. https://doi.org/10.3390/molecules16054131