Three New Phytoecdysteroids Containing a Furan Ring from the Roots of Achyranthes bidentata Bl.
Abstract
:1. Introduction

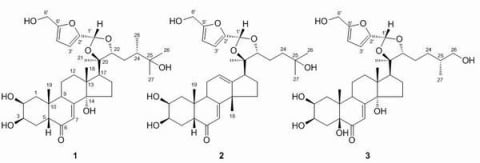
2. Results and Discussion


3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
| No | 1 | 2 | 3 | |||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |||
| 1α | 2.12 (1H, m) | 37.9 | 2.05 (2H, m) | 37.9 | 2.21 (1H, m) | 34.8 | ||
| 1β | 1.90 (1H, m) | 2.09 (1H, m) | ||||||
| 2α | 4.17 (1H, d, 11.8) | 68.2 | 4.17 (1H, m) | 68.0 | 4.23 (1H, m) | 67.9 | ||
| 3α | 4.23 (1H, br. s) | 68.1 | 4.42 (1H, br. s) | 68.3 | 4.18 (1H, m) | 69.9 | ||
| 4α | 1.70 (1H, m) | 32.4 | 1.90 (1H, m) | 32.3 | 1.98 (1H, dd, 14.4, 2.8) | 36.0 | ||
| 4β | 1.97 (1H, m) | 2.25 (1H, m) | 2.10 (1H, m) | |||||
| 5β | 2.98 (1H, dd, 13.2, 3.6) | 51.4 | 2.97 (1H, dd, 13.2, 4.0) | 50.4 | 80.0 | |||
| 6 | 203.4 | 202.4 | 200.9 | |||||
| 7 | 6.24 (1H, d, 2.0) | 121.7 | 6.16 (1H, d, 2.4) | 123.1 | 6.20 (1H, d, 2.4) | 120.0 | ||
| 8 | 165.4 | - | 146.5 | 166.1 | ||||
| 9α | 3.52 (1H, m) | 34.7 | 2.90 (1H, m) | 39.5 | 3.60 (1H, m) | 38.2 | ||
| 10 | 38.6 | 40.3 | 44.7 | |||||
| 11α | 1.78 (1H, m) | 21.0 | 2.19 (1H, m) | 21.7 | 1.88(2H, m) | 21.4 | ||
| 11β | 1.61 (1H, m) | 1.86 (1H, m) | ||||||
| 12α | 2.39 (1H, m) | 31.7 | 6.04 (1H, m) | 122.1 | 1.96 (1H, m) | 31.7 | ||
| 12β | 1.86 (1H, m) | 1.88 (1H, m) | ||||||
| 13 | 47.7 | 173.6 | 47.8 | |||||
| 14 | 84.0 | 48.9 | 83.9 | |||||
| 15α | 2.15 (1H, m) | 31.5 | 1.83 (1H, m) | 38.8 | 2.55 (1H, m) | 31.7 | ||
| 15β | 1.86 (1H, m) | 1.50 (1H, m) | 2.00 (1H, m) | |||||
| 16α | 2.15 (2H, m) | 22.5 | 1.78 (2H, m) | 26.2 | 2.47 (1H, m) | 23.0 | ||
| 16β | 1.98 (1H, m) | |||||||
| 17α | 2.89 (1H, t, 8.6) | 50.6 | 3.11 (1H, t, 9.2) | 49.5 | 2.82 (1H, t, 9.2) | 49.9 | ||
| 18β | 1.00 (3H, s) | 17.3 | 1.09 (3H, s) | 25.4 | 1.12 (3H, s) | 17.3 | ||
| 19β | 1.00 (3H, s) | 24.4 | 0.96 (3H, s) | 23.3 | 0.99 (3H, s) | 17.1 | ||
| 20 | 85.5 | 84.9 | 85.8 | |||||
| 21 | 1.53 (3H, s) | 22.6 | 1.45 (3H, s) | 21.5 | 1.38 (3H, s) | 21.3 | ||
| 22 | 4.20 (1H, dd, 10.4, 3.6) | 85.4 | 4.23 (1H, dd, 10.0, 1.6) | 83.4 | 4.14 (1H, dd, 9.6, 3.2) | 82.9 | ||
| 23 | 2.39 (1H, m) | 31.3 | 2.03 (1H, m) | 26.2 | 1.85 (1H, m) | 27.4 | ||
| 1.86 (1H, m) | 2.16 (1H, m) | 1.52 (1H, m) | ||||||
| 24 | 1.94 (1H, m) | 44.5 | 2.17 (1H, m) | 42.2 | 1.90 (1H, m) | 31.6 | ||
| 1.82 (1H, m) | 1.55 (1H, m) | |||||||
| 25 | 72.1 | 69.2 | 1.82 (1H, m) | 36.7 | ||||
| 26 | 1.28 (3H, s) | 25.4 | 1.41 (3H, s) | 29.6 | 3.63 (2H, m) | 66.8 | ||
| 27 | 1.36 (3H, s) | 28.9 | 1.43 (3H, s) | 30.5 | 1.04 (3H, d, 6.4) | 17.1 | ||
| 28 | 1.19 (3H, d, 6.8) | 16.6 | ||||||
| 1′ | 6.11 (1H, s) | 97.8 | 6.21 (1H, s) | 96.8 | 6.28 (1H, s) | 96.4 | ||
| 2′ | 152.2 | 151.8 | 153.4 | |||||
| 3′ | 6.66 (1H, d, 3.2) | 110.1 | 6.73 (1H, d, 3.2) | 110.3 | 6.61 (1H, d, 3.2) | 109.1 | ||
| 4′ | 6.44 (1H, d, 3.2) | 107.8 | 6.45 (1H, d, 3.2) | 107.8 | 6.43 (1H, d, 3.2) | 107.7 | ||
| 5′ | 157.4 | 157.6 | 157.3 | |||||
| 6′ | 4.83 (2H, s) | 57.2 | 4.85(2H, s) | 57.2 | 4.86 (2H, s) | 57.2 | ||
4. Conclusions
Acknowledgments
References
- Meng, D.L.; Li, X. The research development of Achyranthes bidentata Bl. Chin. J. Med. Chem. 2001, 11, 120–124. [Google Scholar]
- Li, C.C.; Hu, X.G.; Zhang, W.X.; Xie, L.W.; Zhang, H.Y.; Dong, L. Eosinophils apoptosis, fas mRNA and bcl-2 mRNA expressions in asthma model of young rat and effects of Achyranthes bidentata polysaccharides. Zhonghua Er Ke Za Zhi 2003, 41, 657–660. [Google Scholar]
- Chen, X.M.; Xu, Y.J.; Tian, G.Y. Physical-chemical properties and structure elucidation of abPS isolated from the root of Achyranthes bidentata. Yao Xue Xue Bao 2005, 40, 32–35. [Google Scholar]
- Yuan, Y.J.; Cui, Y.; Yu, Y.; Yong, Y.H. Different mechanisms mediate the exciting effect about Achyranthes bidentata on the spike activity of the uterine smooth muscle in virgin rats. Chin. J. Vet. Sci. Technol. 2002, 32, 8–12. [Google Scholar]
- Liu, J.H.; Liang, S.W.; Wang, S.M. A study of antiprocreat effect of Achyranthes bidentata saponin suppository. J. Henan Univ. Chin. Med. 2006, 21, 35–37. [Google Scholar]
- Hu, J.; Qi, Y.X.; Li, Q.X.; Shan, B.E. The research of extract of Achyranthes bidentata Blume anti-tumor activity. Chin. J. Microbiol. Immunol. 2005, 25, 415–418. [Google Scholar]
- Gao, C.K.; Gao, J.; Ma, R.L.; Xu, X.X.; Huang, P.; Ni, S.D. Research on analgesic and anti-inflammatory and invigorate circulation effects of total saponins of Achyranthes. Anhui Med. Pharm. J. 2003, 4, 248–249. [Google Scholar]
- Ma, A.L.; Guo, H. Effect of Achyranthes bidentata on memory and endurance. Zhong Yao Cai 1998, 12, 624–626. [Google Scholar]
- Deng, H.B.; Cui, D.P.; Jiang, J.M.; Feng, Y.C.; Cai, N.S.; Li, D.D. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on nonenzyme glycation in D-galactose induced mouse aging model. Biomed. Environ. Sci. 2003, 16, 267–275. [Google Scholar]
- Gao, C.K. Studies on the preventive and curative effects of Achyranthes bidentata on osteoporosis induced by retinoic acid in rats. Prim. J. Chin. Mater. Med. 2001, 15, 9–11. [Google Scholar]
- Wang, X.J.; Zhu, L.Z. Studies on the saponin constituents of Niu Xi (Achyrathes bidentata). J. Fourth Mil. Med. Univ. 1996, 17, 427–430. [Google Scholar]
- Li, J.X.; Hareyama, T.; Tezuka, Y.; Zhang, Y.; Miyahara, T.; Kadota, S. Five new oleanolic acid glycosides from Achyranthes bidentata with inhibitory activity on osteoclast formation. Planta Med. 2005, 71, 673–679. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.T.; Meng, D.L.; Qiao, A.M. A new phytosterone from the roots of Achyranthes bidentata. Fitoterapia 2007, 78, 607–608, (the correct name of the reported compound is (20R,22R)-2β,3β,20,22,26-pentahydroxy-14β-methyl-18-nor-5β-cholesta-7,12-dien-6-one). [Google Scholar]
- Meng, D.L.; Li, X.; Wang, J.H.; Li, W. A new phytosterone from Achyranthes bidentata Bl. J. Asian Nat. Prod. Res. 2005, 7, 181–184. [Google Scholar] [CrossRef]
- Ecdybase. Available online: http://ecdybase.org/index.php?&action=search (accessed on 30 May 2011).
- Liktor-Busa, E.; Simon, A.; Tóth, G.; Báthori, M. The first two ecdysteroids containing a furan ring from Serratula wolffii. Tetrahedron Lett. 2008, 49, 1738–1740. [Google Scholar] [CrossRef]
- Shigemori, H.; Sato, Y.; Kagata, T.; Kobayashi, J. Palythoalones A and B, new ecdysteroids from the marine zoanthid Palythoa australiae. J. Nat. Prod. 1999, 62, 372–374. [Google Scholar] [CrossRef]
- Miller, R.W.; Clardy, J.; Kozlowski, J.; Mikolajczak, K.L.; Plattne, R.D. Phytoecdysteroids of Diploclisia glaucescens seed. Planta Med. 1985, 51, 40–42. [Google Scholar] [CrossRef]
- Sena Fiho, J.G.; Duringer, J.; Maia, G.L.A.; Tavares, J.F.; Xavier, H.S.; Sobral da Silva, M.; da-Cunha, E.V.L.; Barbosa-Filho, J.M. Ecdysteroids from Vitex species: Distribution and compilation of their 13C-NMR spectral data. Chem. Biodivers. 2008, 5, 707–713. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Kumarihamy, B.M.M.; Arundathie, B.G.S.; Dissanayake, L.; Hara, N.; Fujimoto, Y. A new ecdysteroid, 2-deoxy-5β,20-dihydroxyecdysone from the fruits of Diploclisia glaucescens. Steroids 2003, 68, 447–450. [Google Scholar] [CrossRef]
- Zhu, T.T.; Liang, H.; Zhao, Y.Y.; Wang, B. Isolation and structure identification of C-25 epimers of inokosterone from Achyranthes bidentata Blume. Yao Xue Xue Bao 2004, 39, 913–916. [Google Scholar]
- Baltaev, U.A. Phytoecdysteroids: Structure, sources, and biosynthesis in plants. Russ. J. Bioorg. Chem. 2000, 26, 799–831. [Google Scholar] [CrossRef]
- Ikan, R.; Ravid, U.; Trosset, D.; Shulman, E. Ecdysterone: An insect moulting hormone from Achyranthes aspera (Amaranthaceae). Experientia 1971, 27, 504–505. [Google Scholar]
- Hikino, H.; Hikino, Y.; Nomoto, K.; Takemoto, T. Cyasterone, an insect metamorphosing substance from Cyathula capitata: structure. Tetrahedron 1968, 24, 4895–4906. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takemoto, T.; Ogawa, S.; Nishimoto, N. The moulting hormone activity of ecdysterone and inokosterone isolated from Achyranthis radix. J. Insect Physiol. 1967, 13, 1395–1399. [Google Scholar] [CrossRef]
- Nishimoto, N.; Shiobara, Y.; Inoue, S.S.; Fujino, M.; Takemoto, T.; Yeoh, C.L.; Oliveira, F.D.; Akisue, G.; Akisue, M.K.; Hashimoto, G. Three ecdysteroid glycosides from Pfaffia iresinoides. Phytochemistry 1988, 27, 1665–1668. [Google Scholar] [CrossRef]
- Sarker, S.D.; Girault, J.P.; Lafont, R.; Dinan, L.N. Ecdysteroids from Gomphrena haageana (Amaranthaceae). Biol. Syst. Ecol. 1996, 24, 177–178. [Google Scholar]
- Suksamrarn, A.; Pattanaprateep, P. Selective acetylation of 20-hydroxyecdysone partial synthesis of some minor ecdysteroids and analogues. Tetrahedron 1995, 51, 10633–10650. [Google Scholar] [CrossRef]
- Sample Availability: Samples of niuxixinsterone A, B and C are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Q.-H.; Yang, L.; Jiang, H.; Wang, Z.-B.; Yang, B.-Y.; Kuang, H.-X. Three New Phytoecdysteroids Containing a Furan Ring from the Roots of Achyranthes bidentata Bl. Molecules 2011, 16, 5989-5997. https://doi.org/10.3390/molecules16075989
Wang Q-H, Yang L, Jiang H, Wang Z-B, Yang B-Y, Kuang H-X. Three New Phytoecdysteroids Containing a Furan Ring from the Roots of Achyranthes bidentata Bl. Molecules. 2011; 16(7):5989-5997. https://doi.org/10.3390/molecules16075989
Chicago/Turabian StyleWang, Qiu-Hong, Liu Yang, Hai Jiang, Zhi-Bin Wang, Bing-You Yang, and Hai-Xue Kuang. 2011. "Three New Phytoecdysteroids Containing a Furan Ring from the Roots of Achyranthes bidentata Bl." Molecules 16, no. 7: 5989-5997. https://doi.org/10.3390/molecules16075989
APA StyleWang, Q. -H., Yang, L., Jiang, H., Wang, Z. -B., Yang, B. -Y., & Kuang, H. -X. (2011). Three New Phytoecdysteroids Containing a Furan Ring from the Roots of Achyranthes bidentata Bl. Molecules, 16(7), 5989-5997. https://doi.org/10.3390/molecules16075989