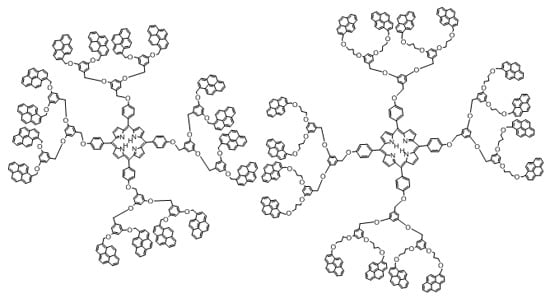
Synthesis of Porphyrin-Dendrimers with a Pyrene in the Periphery and Their Cubic Nonlinear Optical Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Linear and Third Order Non-Linear Optical Characterization
2.2. Crystal Structure Determination
3. Experimental
3.1. General
3.2. Synthesis of Dendrons and Dendrimers
3.2.1. General Procedure
3.2.2. General Procedure
3.2.3. General Procedure
3.3. Cubic NLO-Characterization
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Zeng, F.; Zimmerman, S.C. Dendrimers in supramolecular chemistry: From molecular recognition to self-assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef] [PubMed]
- Newkome, G.R.; He, E.; Moorefield, C.N. Suprasupermolecules with novel properties: Metallodendrimers. Chem. Rev. 1999, 99, 1689–1746. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Diederich, F. Supramolecular dendrimer chemistry: A journey through the branched architecture. Top. Curr. Chem. 2000, 210, 183–227. [Google Scholar]
- Emrick, T.; Fréchet, J.M.J. Self-assembly of dendritic structures. Curr. Opin. Colloid Interf. Sci. 1999, 4, 15–23. [Google Scholar] [CrossRef]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties, and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Dendrimers and other Dendritic Polymers; Fréchet, J.M.J.; Tomalia, D.A. (Eds.) Wiley: Chichester, UK, 2001. [Google Scholar]
- Newkome, G.R.; Moorefield, C.N.; Vögtle, F. Dendrimers and Dendrons: Concepts, Syntheses, Applications; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Valerio, C.; Fillaut, J.L.; Ruiz, J.; Guittard, J.; Blais, J.C.; Astruc, D. The Dendritic effect in molecular recognition: Ferrocene dendrimers and their use as supramolecular redox sensors for the recognition of small inorganic anions. J. Am. Chem. Soc. 1997, 119, 2588–2589. [Google Scholar] [CrossRef]
- Valerio, C.; Alonso, E.; Ruiz, J.; Blais, J.C.; Astruc, D. A polycationic metallodendrimer with 24 [Fe(η5-C5Me5)(η6-N-Alkylaniline)]+ termini that recognizes chloride and bromide anions. Angew. Chem. Int. Ed. 1999, 38, 1747–1751. [Google Scholar] [CrossRef]
- Labande, A.; Astruc, D. Colloids as redox sensors: Recognition of H2PO4− and HSO4− by amidoferrocenylalkylthiol–gold nanoparticles. Chem. Commun. 2000, 1007–1008. [Google Scholar] [CrossRef]
- Nlate, S.; Ruiz, J.; Sartor, V.; Navarro, R.; Blais, J.C.; Astruc, D. Molecular batteries: Ferrocenylsilylation of dendrons, dendritic cores, and dendrimers: New convergent and divergent routes to ferrocenyl dendrimers with stable redox activity. Chem. Eur. J. 2000, 6, 2544–2553. [Google Scholar] [CrossRef]
- Labande, A.; Ruiz, J.; Astruc, D. Supramolecular gold nanoparticles for the redox recognition of oxoanions: Syntheses, titrations, stereoelectronic effects, and selectivity. J. Am. Chem. Soc. 2002, 124, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A., III. Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Ornelas, C.; Ruiz, A.J.; Cloutet, E.; Alves, S.; Astruc, D. Click assembly of 1,2,3-triazole-linked dendrimers, including ferrocenyl dendrimers, which sense both oxo anions and metal cations. Angwe. Chem. Int. Ed. 2007, 46, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Goodson, T., III; Varnavski, O.; Wang, Y. Optical properties and applications of dendrimer-metal nanocomposites. Int. Rev. Phys. Chem. 2004, 23, 109–150. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H.; Kaifer, E.A. Redox active, hybrid dendrimers containing Fréchet- and Newkome-type blocks. Org. Lett. 2007, 9, 2657–2660. [Google Scholar] [CrossRef] [PubMed]
- Ispasoiu, R.G.; Balogh, L.; Varnavski, O.P.; Tomalia, D.A.; Goodson, T., III. Large optical limiting from novel metal-dendrimer nanocomposite materials. J. Am. Chem. Soc. 2000, 122, 11005–11006. [Google Scholar] [CrossRef]
- Samoc, M.; Samoc, A.; Luther-Davies, B.; Humphrey, M.G.; Wong, M.S. Third-order optical nonlinearities of oligomers, dendrimers and polymers derived from solution Z-scan studies. Opt. Mater. 2003, 21, 485–488. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Boas, U.; Christensen, J.B.; Heegaard, P.M.H. Dendrimers in Medicine and Biotechnology: New Molecular Tools; RSC Publishing: Cambridge, UK, 2006. [Google Scholar]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.E.; Hurst, S.K.; Morrall, J.P.; Cifuentes, M.P.; Roberts, R.L.; Samoc, M.; Humphrey, M.G. Organometallic complexes for nonlinear optics. 39. Syntheses and third-order nonlinear optical properties of first-generation peripherally metalated arylalkynyl dendrimers. Organometallics 2007, 26, 4456–5576. [Google Scholar] [CrossRef]
- Majoral, J.P. State of the art developments in the chemistry and properties of dendrimers and hyperbranched polymers. New J. Chem. 2007, 31, 1039–1040. [Google Scholar]
- Al-Jamal, K.T.; Ramaswamy, C.; Florence, A.T. Supramolecular structures from dendrons and dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2238–2270. [Google Scholar] [CrossRef] [PubMed]
- Röglin, L.; Lempens, E.H.M.; Meijer, E.W. A synthetic “tour de force”: Well-defined multivalent and multimodal dendritic structures for biomedical applications. Angew. Chem. Int. Ed. 2011, 50, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.P.; Powell, C.E.; Morrall, J.P. Electrochemical, spectroelectrochemical, and molecular quadratic and cubic nonlinear optical properties of alkynylruthenium dendrimers. J. Am. Chem. Soc. 2006, 128, 10819–10832. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.; Grimsdale, A.C.; Holmes, A.B. Electroluminescent conjugated polymers-seeing polymers in a new light. Angew. Chem. Int. Ed. 1998, 37, 402–428. [Google Scholar] [CrossRef]
- Lo, S.-C.; Burn, P.L. Development of dendrimers: Macromolecules for use in organic light-emitting diodes and solar cells. Chem. Rev. 2007, 107, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Burn, P.L.; Lo, S.-C.; Samuel, I.D.W. Metallo-supramolecular block copolymers. Adv. Mater. 2007, 19, 1675–1688. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7648. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; van Stryland, E.W. High-sensitivity, single-beam n2 measurements. Opt. Lett. 1989, 14, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Sheik-Bahae, M.; Said, A.A.; Hagan, D.J.; Soileau, M.J.; van Stryland, E.W. Nonlinear refraction and optical limiting in “thick” media. Opt. Eng. 1991, 30, 1228–1235. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.-H.; Hagan, D.J.; van Stryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- Xia, T.; Hagan, D.J.; Sheik-Bahae, M.; van Stryland, E.W. Eclipsing Z-scan measurement of λ/104 wave-front distortion. Opt. Lett. 1994, 19, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Nonlinear Optics of Organic Molecules and Polymers; Nalwa, H.S.; Miyata, S. (Eds.) CRS Press Inc.: Boca Raton, FL, USA, 1997. [Google Scholar]
- Liu, X.; Guo, S.; Wang, H.; Hou, L. Theoretical study on the closed-aperture Z-scan curves in the materials with nonlinear refraction and strong nonlinear absorption. Opt. Commun. 2001, 197, 431–437. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, A.A.; Morales-Saavedra, O.G.; Román, C.J.; Ortega-Martínez, R. Variation of nonlinear refractive index in dye-doped liquid crystals by local and nonlocal mechanisms. Opt. Mater. 2008, 31, 350–360. [Google Scholar] [CrossRef]
Sample Availability: Not available |









| Compound | λmax, (nm) |
|---|---|
| 15 | 329, 345, 423,518, 557, 597, 653. |
| 17 | 315, 329, 345, 375, 423, 519, 555, 595, 652 |
| 16 | 243, 277, 328, 344, 422, 453, 518, 551, 595, 651, 689 |
| 18 | 244, 278, 329, 345, 423, 455, 519, 557, 594, 652, 693 |
| Dendron/Dendrimer Film Sample | Linear Refractive Index: n0 @ λZ-Scan = 633 nm | Linear Absorption Coefficient: α0 (@ 633 nm) [m−1] | Sample Thickness [nm] | Δφ0/Δψ0 | NLO-Refractive Index: γ/n2 Z-Scan @ λ = 632nm × 10−8 [m2 W−1]/ × 102 [esu] | NLO-Absorption: β (TPA or SA) [×10−2 m W−1] |
|---|---|---|---|---|---|---|
| Dendron 9 | 1.56 ± 0.052 | 54,185.5 | 2080 | 0.0/0.0 | 0.0/0.0 | 0.0 |
| Dendron 13 | 1.59 ± 0.055 | 117,828.2 | 6590 | +4.2/+0.05 | +0.684/+2.56 | +0.162 (TPA) |
| Dendrimer 16 | 1.68 ± 0.047 | 524,837.9 | 124 | +0.4/0.0 | +2.49/+9.27 | 0.0 |
| Dendrimer 18 | 1.74 ± 0.045 | 1,284,314 | 116 | +3.0/+0.05 | +27.7/+104 | +9.15 (TPA) |
| Empirical formula | C17H12O |
| Formula weight | 232.27 |
| Temperature | 298(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| Unit cell dimensions | a = 19.995(3) Å |
| b = 8.9672(14) Å | |
| c = 13.232(2) Å | |
| Volume | 2370.8(7) Å3 |
| Z | 8 |
| Density (calculated) | 1.301 Mg/m3 |
| Absorption coefficient | 0.079 mm−1 |
| F(000) | 976 |
| Crystal size/shape/color | 0.32 × 0.23 × 0.10 mm/Prism/Colorless |
| Theta range for data collection | 2.04 to 25.38° |
| Index ranges | −24 ≤ h ≤24, −10 ≤ k ≤10, −15 ≤ l ≤15 |
| Reflections collected | 19076 |
| Independent reflections | 4355 [R(int) = 0.0904] |
| Completeness to theta = 25.36° | 99.9% |
| Absorption correction | None |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 4355/2/331 |
| Goodness-of-fit on F2 | 0.804 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0473, wR2 = 0.0740 |
| R indices (all data) | R1 = 0.1546, wR2 = 0.0954 |
| Largest diff. peak and hole | 0.124 and −0.092 e.Å−3 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morales-Espinoza, E.G.; Lijanova, I.V.; Morales-Saavedra, O.G.; Torres-Zuñiga, V.; Hernandez-Ortega, S.; Martínez-García, M. Synthesis of Porphyrin-Dendrimers with a Pyrene in the Periphery and Their Cubic Nonlinear Optical Properties. Molecules 2011, 16, 6950-6968. https://doi.org/10.3390/molecules16086950
Morales-Espinoza EG, Lijanova IV, Morales-Saavedra OG, Torres-Zuñiga V, Hernandez-Ortega S, Martínez-García M. Synthesis of Porphyrin-Dendrimers with a Pyrene in the Periphery and Their Cubic Nonlinear Optical Properties. Molecules. 2011; 16(8):6950-6968. https://doi.org/10.3390/molecules16086950
Chicago/Turabian StyleMorales-Espinoza, Eric G., Irina V. Lijanova, Omar G. Morales-Saavedra, Vícente Torres-Zuñiga, Simon Hernandez-Ortega, and Marcos Martínez-García. 2011. "Synthesis of Porphyrin-Dendrimers with a Pyrene in the Periphery and Their Cubic Nonlinear Optical Properties" Molecules 16, no. 8: 6950-6968. https://doi.org/10.3390/molecules16086950
APA StyleMorales-Espinoza, E. G., Lijanova, I. V., Morales-Saavedra, O. G., Torres-Zuñiga, V., Hernandez-Ortega, S., & Martínez-García, M. (2011). Synthesis of Porphyrin-Dendrimers with a Pyrene in the Periphery and Their Cubic Nonlinear Optical Properties. Molecules, 16(8), 6950-6968. https://doi.org/10.3390/molecules16086950