Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats
Abstract
:1. Introduction

2. Results and Discussion
2.1. Results
2.1.1. Method Validation

| Tissues | Compounds | Regression equation | Correlation coefficient (r2) | Linear range (µg/mL) |
|---|---|---|---|---|
| Heart | THP | Y = 2.1542X + 0.0544 | 0.9997 | 0.0306~0.612 |
| DHC | Y = 10.686X + 0.0350 | 0.9997 | 0.0294~0.588 | |
| Protopine | Y = 2.2532X + 0.0191 | 0.9996 | 0.0320~0.640 | |
| Liver | THP | Y = 2.7012X + 0.0026 | 0.9983 | 0.0306~3.06 |
| DHC | Y = 13.784X − 0.1516 | 0.9990 | 0.0294~2.94 | |
| Protopine | Y = 2.9222X + 0.0080 | 0.9992 | 0.0320~3.20 | |
| Spleen | THP | Y = 2.6201X + 0.0310 | 0.9987 | 0.0306~3.06 |
| DHC | Y = 12.847X + 0.0475 | 0.9984 | 0.0294~2.94 | |
| Protopine | Y = 2.7289X + 0.0966 | 0.9990 | 0.0320~3.20 | |
| Lung | THP | Y = 2.7128X + 0.0130 | 0.9980 | 0.0306~3.06 |
| DHC | Y = 12.801X + 0.0007 | 0.9994 | 0.0294~2.94 | |
| Protopine | Y = 2.8704X − 0.0077 | 0.9984 | 0.0320~3.20 | |
| Kidney | THP | Y = 2.5418X + 0.0957 | 0.9981 | 0.0306~3.06 |
| DHC | Y = 12.299X + 0.2340 | 0.9980 | 0.0294~2.94 | |
| Protopine | Y = 2.6138X + 0.0709 | 0.9989 | 0.0320~3.20 | |
| Brain | THP | Y = 2.5324X + 0.0217 | 0.9999 | 0.0306~0.306 |
| DHC | Y = 12.657X − 0.0744 | 0.9998 | 0.0294~0.294 | |
| Protopine | Y = 2.5671X + 0.0143 | 0.9997 | 0.0320~0.320 |
2.1.2. Effect of Vinegar and Wine Processing on the Tissue Distribution of THP

| Tissue | Tmax | Cmax (µg/mL) | AUC0–24 h (µg·h/mL) | MRT(h) |
|---|---|---|---|---|
| Heart | ||||
| Crude extract | 0.25 ± 0.01b | 0.59 ± 0.12a | 1.44 ± 0.25a | 4.61 ± 0.08b |
| Vinegar processed | 0.25 ± 0.03b | 0.66 ± 0.07a | 1.63 ± 0.28a | 4.64 ± 0.11b |
| Wine processed | 0.51 ± 0.01a | 0.51 ± 0.11a | 1.22 ± 0.25a | 5.40 ± 0.19a |
| Pure compound | 0.25 ± 0.00b | 0.60 ± 0.18a | 1.43 ± 0.32a | 4.83 ± 0.13b |
| Liver | ||||
| Crude extract | 0.25 ± 0.00b | 4.22 ± 0.87a | 6.91 ± 2.08a | 4.52 ± 0.08a |
| Vinegar processed | 0.26 ± 0.02b | 6.07 ± 0.64a | 6.04 ± 2.87a | 4.28 ± 0.07a |
| Wine processed | 0.53 ± 0.04a | 3.72 ± 0.78a | 5.86 ± 1.22a | 3.78 ± 0.13b |
| Pure compound | 0.21 ± 0.01b | 7.84 ± 1.74a | 5.57 ± 2.60a | 3.99 ± 0.08b |
| Spleen | ||||
| Crude extract | 0.25 ± 0.05b | 1.26 ± 0.27a | 3.88 ± 0.67a | 4.78 ± 0.10b |
| Vinegar processed | 0.27 ± 0.01b | 1.77 ± 0.19a | 4.71 ± 0.85a | 4.23 ± 0.07c |
| Wine processed | 0.52 ± 0.04a | 1.13 ± 0.24a | 3.53 ± 0.71a | 6.21 ± 0.24a |
| Pure compound | 0.23 ± 0.00b | 1.54 ± 0.45a | 3.27 ± 0.74a | 4.34 ± 0.09c |
| Lung | ||||
| Crude extract | 0.25 ± 0.01b | 1.44 ± 0.30a | 5.33 ± 0.91a | 5.74 ± 0.13b |
| Vinegar processed | 0.28 ± 0.01b | 1.68 ± 0.18a | 7.02 ± 1.18a | 5.27 ± 0.23b |
| Wine processed | 0.51 ± 0.03a | 1.04 ± 0.22a | 6.56 ± 0.72a | 6.23 ± 0.27a |
| Pure compound | 0.26 ± 0.08b | 1.53 ± 0.45a | 5.93 ± 1.32a | 5.34 ± 0.15b |
| Kidney | ||||
| Crude extract | 0.51 ± 0.00a | 1.66 ± 0.33a | 4.69 ± 0.83a | 5.17 ± 0.13b |
| Vinegar processed | 0.53 ± 0.02a | 2.12 ± 0.42a | 6.78 ± 1.27a | 4.50 ± 0.08c |
| Wine processed | 0.58 ± 0.05a | 1.45 ± 0.31a | 4.32 ± 0.87a | 5.65 ± 0.21a |
| Pure compound | 0.33 ± 0.09b | 2.05 ± 0.57a | 6.27 ± 1.43a | 4.37 ± 0.10c |
| Cerebrum | ||||
| Crude extract | 0.25 ± 0.01b | 0.22 ± 0.046a | 0.75 ± 0.13a | 7.82 ± 0.26b |
| Vinegar processed | 0.28 ± 0.03b | 0.26 ± 0.026a | 0.95 ± 0.17a | 7.24 ± 0.24b |
| Wine processed | 0.51 ± 0.07a | 0.19 ± 0.04a | 0.66 ± 0.13a | 9.39 ± 0.46a |
| Pure compound | 0.26 ± 0.01b | 0.25 ± 0.072a | 0.86 ± 0.20a | 8.0 ± 0.25b |
| Cerebellum | ||||
| Crude extract | 0.25 ± 0.02b | 0.21 ± 0.04a | 0.96 ± 0.16a | 7.50 ± 0.23c |
| Vinegar processed | 0.27 ± 0.03b | 0.24 ± 0.03a | 1.14 ± 0.20a | 8.28 ± 0.36bc |
| Wine processed | 0.55 ± 0.01a | 0.19 ± 0.04a | 0.87 ± 0.17a | 8.52 ± 0.46ab |
| Pure compound | 0.26 ± 0.02b | 0.15 ± 0.03a | 0.99 ± 0.22a | 9.20 ± 0.61a |
| Diencephalons | ||||
| Crude extract | 0.23 ± 0.01b | 0.45 ± 0.10a | 1.70 ± 0.29a | 7.76 ± 0.23b |
| Vinegar processed | 0.25 ± 0.01b | 0.51 ± 0.06a | 1.88 ± 0.33a | 7.31 ± 0.29b |
| Wine processed | 0.52 ± 0.03a | 0.40 ± 0.09a | 1.38 ± 0.27a | 9.06 ± 0.57a |
| Pure compound | 0.27 ± 0.04b | 0.46 ± 0.13a | 1.67 ± 0.38a | 7.70 ± 0.28b |
| Brainstem | ||||
| Crude extract | 0.25 ± 0.02b | 0.34 ± 0.07a | 1.33 ± 0.23a | 6.82 ± 0.21b |
| Vinegar processed | 0.28 ± 0.01b | 0.41 ± 0.04a | 1.66 ± 0.30a | 7.20 ± 0.25b |
| Wine processed | 0.52 ± 0.01a | 0.28 ± 0.06a | 1.11 ± 0.22a | 9.12 ± 0.47a |
| Pure compound | 0.25 ± 0.00b | 0.37 ± 0.11a | 1.49 ± 0.34a | 7.53 ± 0.26b |
| Hippocampus | ||||
| Crude extract | 0.22 ± 0.03a | 0.47 ± 0.10a | 1.57 ± 0.27a | 6.55 ± 0.20a |
| Vinegar processed | 0.25 ± 0.01a | 0.52 ± 0.05a | 1.98 ± 0.35a | 6.78 ± 0.29a |
| Wine processed | 0.27 ± 0.02a | 0.39 ± 0.11a | 1.49 ± 0.30a | 6.78 ± 0.30a |
| Pure compound | 0.24 ± 0.05a | 0.47 ± 0.13a | 1.79 ± 0.40a | 7.04 ± 0.23a |
| Striatum | ||||
| Crude extract | 0.25 ± 0.02a | 0.72 ± 0.15a | 2.40 ± 0.42a | 6.30 ± 0.18b |
| Vinegar processed | 0.22 ± 0.07a | 0.79 ± 0.09a | 2.77 ± 0.49a | 6.33 ± 0.28b |
| Wine processed | 0.28 ± 0.01a | 0.64 ± 0.18a | 2.10 ± 0.42a | 5.34 ± 0.17c |
| Pure compound | 0.21 ± 0.03a | 0.72 ± 0.21a | 2.47 ± 0.57a | 7.69 ± 0.26a |
2.1.3. Effect of Vinegar and Wine Processing on the Tissue Distribution of DHC
| Tissue | Tmax (h) | Cmax (µg/mL) | AUC0–24 h (µg·h/mL) | MRT (h) |
|---|---|---|---|---|
| Heart | ||||
| Crude extract | 0.25 ± 0.01b | 0.68 ± 0.14a | 1.33 ± 0.23a | 4.20 ± 0.07a |
| Vinegar processed | 0.51 ± 0.00a | 0.54 ± 0.10a | 0.97 ± 0.18a | 5.05 ± 0.15a |
| Wine processed | 0.53 ± 0.01a | 0.47 ± 0.10a | 0.99 ± 0.20a | 6.12 ± 0.36a |
| Pure compound | 0.25 ± 0.00b | 0.44 ± 0.13a | 0.85 ± 0.12a | 8.72 ± 6.38a |
| Liver | ||||
| Crude extract | 1.12 ± 0.02a | 1.03 ± 0.21a | 2.90 ± 0.51a | 4.48 ± 0.12bc |
| Vinegar processed | 0.53 ± 0.03b | 0.85 ± 0.17a | 2.13 ± 0.39ab | 6.43 ± 0.20a |
| Wine processed | 0.25 ± 0.00c | 0.88 ± 0.25a | 1.89 ± 0.40ab | 4.19 ± 0.13c |
| Pure compound | 0.25 ± 0.00c | 0.62 ± 0.18a | 1.57 ± 0.34b | 4.85 ± 0.10b |
| Spleen | ||||
| Crude extract | 1.01 ± 0.07a | 0.53 ± 0.10a | 1.13 ± 0.20a | 5.06 ± 0.10a |
| Vinegar processed | 0.50 ± 0.06b | 0.46 ± 0.09a | 0.78 ± 0.14a | 3.97 ± 0.11b |
| Wine processed | 0.58 ± 0.00b | 0.40 ± 0.09a | 0.83 ± 0.17a | 3.50 ± 0.10c |
| Pure compound | 0.25 ± 0.10c | 0.36 ± 0.08a | 0.83 ± 0.19a | 3.26 ± 0.08c |
| Lung | ||||
| Crude extract | 0.50 ± 0.00a | 0.58 ± 0.12a | 1.32 ± 0.23a | 5.44 ± 0.15a |
| Vinegar processed | 0.54 ± 0.02a | 0.50 ± 0.10a | 0.84 ± 0.16ab | 4.34 ± 0.11b |
| Wine processed | 0.51 ± 0.09a | 0.43 ± 0.10a | 0.98 ± 0.20ab | 4.61 ± 0.15b |
| Pure compound | 0.25 ± 0.01b | 0.37 ± 0.11a | 0.57 ± 0.13b | 3.98 ± 0.10c |
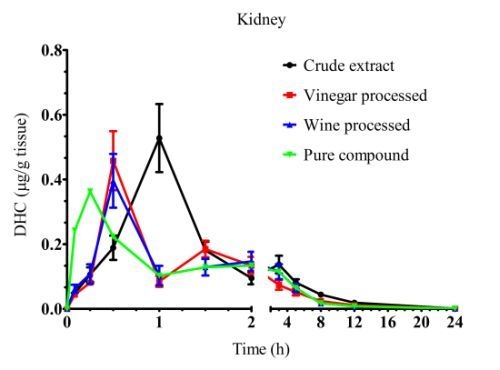
| Kidney | ||||
| Crude extract | 0.25 ± 0.02c | 0.51 ± 0.05ab | 1.51 ± 0.05a | 5.01 ± 0.07a |
| Vinegar processed | 0.53 ± 0.01b | 0.40 ± 0.03b | 1.22 ± 0.08a | 5.05 ± 0.03a |
| Wine processed | 1.51 ± 0.01a | 0.40 ± 0.03b | 0.94 ± 0.07a | 4.17 ± 0.29b |
| Pure compound | 0.25 ± 0.01c | 0.31 ± 0.02b | 0.89 ± 0.09b | 4.94 ± 0.12a |
| Cerebrum | ||||
| Crude extract | 0.25 ± 0.00b | 0.22 ± 0.24a | 0.22 ± 0.03a | 5.18 ± 0.10b |
| Vinegar processed | 1.55 ± 0.03a | 0.05 ± 0.01a | 0.19 ± 0.03a | 4.90 ± 0.10b |
| Wine processed | 1.50 ± 0.01a | 0.06 ± 0.01a | 0.19 ± 0.04a | 6.79 ± 0.34a |
| Pure compound | 0.25 ± 0.02b | 0.05 ± 0.01a | 0.15 ± 0.03a | 5.39 ± 0.19ab |
| Cerebellum | ||||
| Crude extract | 0.25 ± 0.05c | 0.07 ± 0.01a | 0.29 ± 0.06a | 6.16 ± 0.19a |
| Vinegar processed | 1.09 ± 0.03a | 0.06 ± 0.011a | 0.20 ± 0.03ab | 6.25 ± 0.30a |
| Wine processed | 0.58 ± 0.08b | 0.06 ± 0.01a | 0.18 ± 0.04ab | 6.82 ± 1.08a |
| Pure compound | 0.25 ± 0.00c | 0.05 ± 0.02a | 0.17 ± 0.03b | 5.67 ± 0.32a |
| Diencephalons | ||||
| Crude extract | 2.12 ± 0.08a | 0.07 ± 0.02a | 0.35 ± 0.06a | 9.22 ± 0.40a |
| Vinegar processed | 1.07 ± 0.12b | 0.09 ± 0.02a | 0.26 ± 0.04ab | 8.10 ± 0.29b |
| Wine processed | 0.53 ± 0.04c | 0.08 ± 0.02a | 0.20 ± 0.04b | 4.96 ± 0.15c |
| Pure compound | 0.52 ± 0.11c | 0.08 ± 0.02a | 0.21 ± 0.05b | 5.30 ± 0.12c |
| Brain stem | ||||
| Crude extract | 0.51 ± 0.02b | 0.22 ± 0.04a | 0.60 ± 0.10a | 4.21 ± 0.10c |
| Vinegar processed | 1.53 ± 0.05a | 0.17 ± 0.03a | 0.58 ± 0.11a | 6.25 ± 0.16a |
| Wine processed | 0.54 ± 0.01b | 0.17 ± 0.03a | 0.50 ± 0.10a | 5.46 ± 0.13b |
| Pure compound | 0.50 ± 0.00b | 0.16 ± 0.03a | 0.41 ± 0.06a | 4.29 ± 0.15c |
| Hippocampus | ||||
| Crude extract | 1.51 ± 0.23b | 0.09 ± 0.01a | 0.37 ± 0.06a | 8.15 ± 0.21a |
| Vinegar processed | 1.54 ± 0.06b | 0.07 ± 0.01a | 0.28 ± 0.05a | 8.68 ± 0.76a |
| Wine processed | 2.07 ± 0.21a | 0.07 ± 0.02a | 0.28 ± 0.05a | 9.35 ± 0.88a |
| Pure compound | 1.08 ± 0.07c | 0.07 ± 0.02a | 0.27 ± 0.06a | 8.43 ± 0.51a |
| Striatum | ||||
| Crude extract | 0.25 ± 0.00c | 0.09 ± 0.02a | 0.20 ± 0.07a | 7.15 ± 0.22a |
| Vinegar processed | 1.01 ± 0.03a | 0.06 ± 0.02a | 0.24 ± 0.04a | 6.70 ± 0.43a |
| Wine processed | 0.52 ± 0.04b | 0.06 ± 0.01a | 0.22 ± 0.04a | 5.19 ± 0.11b |
| Pure compound | 0.25 ± 0.01c | 0.06 ± 0.02a | 0.21 ± 0.04a | 7.27 ± 0.30a |

2.1.4. Effect of Vinegar and Wine Processing on the Tissue Distribution of Protopine
| Tissue | Tmax (h) | Cmax (µg/mL) | AUC0–24 h (µg·h/mL) | MRT (h) |
|---|---|---|---|---|
| Heart | ||||
| Crude extract | 5.11 ± 0.24a | 1.51 ± 0.18a | 6.26 ± 0.92a | 5.93 ± 0.02a |
| Vinegar processed | 5.07 ± 0.15a | 1.59 ± 0.43a | 5.61 ± 1.06a | 5.70 ± 0.03b |
| Wine processed | 3.55 ± 0.31b | 1.49 ± 0.30a | 4.22 ± 0.85a | 4.85 ± 0.03c |
| Pure compound | 5.28 ± 0.42a | 1.50 ± 0.45a | 6.23 ± 1.56a | 5.93 ± 0.02a |
| Liver | ||||
| Crude extract | 5.32 ± 0.12a | 6.78 ± 0.78a | 29.16 ± 4.24a | 5.46 ± 0.01b |
| Vinegar processed | 5.25 ± 0.16a | 6.91 ± 1.42a | 28.13 ± 5.30a | 5.28 ± 0.02b |
| Wine processed | 3.07 ± 0.10b | 6.91 ± 1.40a | 19.30 ± 3.84a | 5.46 ± 0.17ab |
| Pure compound | 5.09 ± 0.68a | 6.67 ± 1.97a | 27.83 ± 6.98a | 5.69 ± 0.03a |
| Spleen | ||||
| Crude extract | 5.87 ± 0.32a | 7.55 ± 0.87a | 34.91 ± 5.19a | 5.59 ± 0.03a |
| Vinegar processed | 5.12 ± 0.34a | 7.05 ± 1.45a | 33.36 ± 6.42a | 5.29 ± 0.04b |
| Wine processed | 3.07 ± 0.61b | 7.03 ± 1.41a | 20.45 ± 4.13a | 3.86 ± 0.03b |
| Pure compound | 5.22 ± 0.05a | 7.42 ± 2.22a | 35.03 ± 8.18a | 5.65 ± 0.03a |
| Lung | ||||
| Crude extract | 5.14 ± 0.26a | 11.42 ± 1.42a | 51.16 ± 7.69a | 5.86 ± 0.03b |
| Vinegar processed | 5.63 ± 0.02a | 11.78 ± 2.41a | 50.69 ± 9.57a | 5.97 ± 0.01a |
| Wine processed | 3.87 ± 0.05b | 10.38 ± 2.08a | 31.54 ± 7.27a | 4.76 ± 0.03c |
| Pure compound | 5.22 ± 0.47a | 11.22 ± 3.31a | 50.24 ± 12.46a | 5.85 ± 0.04b |
| Kidney | ||||
| Crude extract | 5.01 ± 0.06a | 4.49 ± 0.52a | 18.65 ± 2.79a | 5.54 ± 0.04a |
| Vinegar processed | 5.09 ± 0.38a | 4.37 ± 0.90a | 18.48 ± 3.49a | 5.32 ± 0.04b |
| Wine processed | 3.10 ± 0.28b | 4.60 ± 0.91a | 13.78 ± 2.72a | 4.93 ± 0.03c |
| Pure compound | 5.13 ± 0.04a | 4.42 ± 1.31a | 13.44 ± 2.65a | 5.53 ± 0.04a |
| Cerebrum | ||||
| Crude extract | 2.09 ± 0.02b | 1.46 ± 0.29a | 3.87 ± 0.69a | 4.22 ± 0.22b |
| Vinegar processed | 3.11 ± 0.22a | 1.15 ± 0.25a | 5.02 ± 0.97a | 6.37 ± 0.03a |
| Wine processed | 2.08 ± 0.02b | 1.50 ± 0.30a | 4.57 ± 0.91a | 6.21 ± 0.03a |
| Pure compound | 2.10 ± 0.80b | 1.35 ± 0.27a | 3.65 ± 0.78a | 3.93 ± 0.06b |
| Cerebellum | ||||
| Crude extract | 2.13 ± 0.05b | 1.69 ± 0.34a | 4.41 ± 0.72a | 5.56 ± 0.15a |
| Vinegar processed | 3.28 ± 0.17a | 1.47 ± 0.29a | 4.93 ± 0.93a | 4.94 ± 0.01b |
| Wine processed | 2.21 ± 0.38b | 1.65 ± 0.33a | 4.67 ± 0.94a | 4.98 ± 0.02b |
| Pure compound | 2.08 ± 0.01b | 1.56 ± 0.32a | 3.80 ± 0.83a | 4.86 ± 0.08b |
| Diencephalons | ||||
| Crude extract | 2.11 ± 0.22b | 1.37 ± 0.28a | 3.48 ± 0.59a | 4.94 ± 0.14c |
| Vinegar processed | 3.08 ± 0.15a | 1.14 ± 0.23a | 3.98 ± 0.76a | 5.76 ± 0.03a |
| Wine processed | 2.03 ± 0.02b | 1.43 ± 0.28a | 3.91 ± 0.79a | 5.42 ± 0.03b |
| Pure compound | 2.07 ± 0.21b | 1.31 ± 0.27a | 3.18 ± 0.07a | 4.89 ± 0.01c |
| Brain stem | ||||
| Crude extract | 2.00 ± 0.08b | 0.91 ± 0.19a | 2.42 ± 0.46a | 4.50 ± 0.18b |
| Vinegar processed | 3.13 ± 0.41a | 0.89 ± 0.18a | 2.58 ± 0.46a | 4.90 ± 0.19a |
| Wine processed | 2.09 ± 0.10b | 1.27 ± 0.21a | 2.80 ± 0.57a | 5.13 ± 0.03a |
| Pure compound | 2.20 ± 0.01b | 0.82 ± 0.17a | 2.02 ± 0.42a | 3.90 ± 0.14b |
| Hippocampus | ||||
| Crude extract | 2.10 ± 0.03b | 1.77 ± 0.36a | 4.24 ± 0.87a | 5.02 ± 0.15b |
| Vinegar processed | 3.19 ± 0.18a | 1.58 ± 0.31a | 5.31 ± 0.97a | 6.07 ± 0.16a |
| Wine processed | 2.28 ± 0.01b | 1.75 ± 0.35a | 4.80 ± 0.96a | 4.37 ± 0.03c |
| Pure compound | 2.09 ± 0.04b | 1.56 ± 0.31a | 3.66 ± 0.81a | 5.23 ± 0.10b |
| Striatum | ||||
| Crude extract | 2.01 ± 0.01b | 2.18 ± 0.44a | 6.51 ± 1.44a | 6.90 ± 0.20a |
| Vinegar processed | 3.02 ± 0.07a | 1.85 ± 0.37a | 7.23 ± 1.29a | 5.51 ± 0.26b |
| Wine processed | 2.31 ± 0.02b | 2.17 ± 0.44a | 7.64 ± 1.50a | 6.52 ± 0.01a |
| Pure compound | 2.07 ± 0.08b | 1.95 ± 0.40a | 6.42 ± 1.52a | 6.57 ± 0.43a |

2.2. Discussion
3. Experimental
3.1. Chemicals
3.2. Animals
3.3. Preparation of Rhizoma Corydalis Extract
3.4. Administration and Tissue Sample Collection
3.5. Extraction Procedures
3.6. Simultaneous Determination of THP, DHC and Protopine in Rat Tissues
3.7. Validation of the Method for Quantitative Analysis of THP, DHC and Protopine in Rat Tissues
3.8. Pharmacokinetic and Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Yuan, C.S.; Mehendale, S.R.; Wang, C.Z.; Aung, H.H.; Jiang, T.L.; Guan, X.F.; Shoyama, Y. Effects of Corydalis yanhusuo and Angelicae dahuricae on cold pressor-induced pain in humans: A controlled trial. J. Clin. Pharmcol. 2004, 44, 1323–1327. [Google Scholar] [CrossRef]
- Ding, B.; Zhou, T.T.; Fan, G.R.; Hong, Z.Y.; Wu, Y.T. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo WT Wang by LC-MS/MS and LC-DAD. J. Pharm. Biomed. Anal. 2007, 45, 219–226. [Google Scholar] [CrossRef]
- Lee, Y.L.; Sagare, A.P.; Lee, C.Y.; Feng, H.T.; Ko, Y.C.; Shaw, J.F.; Tsay, H.S. Formation of protoberberine-type alkaloids by the tubers of somatic embryo-derived plants of Corydalis yanhusuo. Planta Med. 2001, 67, 839–842. [Google Scholar] [CrossRef]
- Chu, H.Y.; Jin, G.Z.; Friedman, E.; Zhen, X.C. Recent development in studies of tetrahydroprotoberberines: Mechanism in antinociception and drug addiction. Cell. Mol. NeuroBiol. 2008, 28, 491–499. [Google Scholar] [CrossRef]
- Oh, Y.C.; Choi, J.G.; Lee, Y.S.; Brice, O.O.; Lee, S.C.; Kwak, H.S.; Byun, Y.H.; Kang, O.H.; Rho, J.R.; Shin, D.W.; Kwon, D.Y. Tetrahydropalmatine Inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated THP-1 Cells. J. Med. Food 2010, 13, 1125–1132. [Google Scholar] [CrossRef]
- Gao, J.L.; He, T.C.; Li, Y.B.; Wang, Y.T. A traditional Chinese medicine formulation consisting of Rhizoma Corydalis and Rhizoma Curcumae exerts synergistic anti-tumor activity. Oncol. Rep. 2009, 22, 1077–1083. [Google Scholar]
- Shi, J.; Zhang, X.; Ma, Z.; Zhang, M.; Sun, F. Characterization of aromatase binding agents from the dichloromethane extract of Corydalis yanhusuo using ultrafiltration and liquid chromatography tandem mass spectrometry. Molecules 2010, 15, 3556–3566. [Google Scholar] [CrossRef]
- Wu, L.M.; Ling, H.Y.; Li, L.D.; Jiang, L.X.; He, M.L. Beneficial effects of the extract from Corydalis yanhusuo in rats with heart failure following myocardial infarction. J. Pharm. Pharmacol. 2007, 59, 695–701. [Google Scholar]
- Li, P.; Ren, J.; Duan, C.; Lin, C.; Liu, J. Effects of four components of Rhizoma Corydalis on anoxia and peroxidation injuries in neonatal cardiomyocytes. Zhongguo Zhong Yao Za Zhi 2010, 35, 84–88. [Google Scholar]
- Ling, H.; Wu, L.; Li, L. Corydalis yanhusuo Rhizoma extract reduces infarct size and improves heart function during myocardial ischemia/reperfusion by inhibiting apoptosis in rats. Phytother. Res. 2006, 20, 448–453. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, J.; Hu, J.; Li, T.; Zhang, Y. Protective effect of protopine on the focal cerebral ischaemic injury in rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 85–89. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Fan, Z.C.; Xie, C.J. Simultaneous quantitation of tetrahydropalmatine and protopine in rabbit plasma by HPLC-PAD, and application to pharmacokinetic studies. Chromatographia 2006, 64, 577–581. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Zhang, F.; Wang, Y. RP-HPLC simultaneous determination of tetrahydropalmatine and protopine in Rhizoma Corydalis. J. Shaanxi Norm. Univ. 2005, 33, 1–23. [Google Scholar]
- Zhu, Y.P. Chinese Materia Medica: Chemistry,Pharmacology and Applications; Harwood Academic Publishers: Amsterdam,The Netherlands, 1998; pp. 17–20. [Google Scholar]
- Pharmacopoeia Commission, Pharmacopoeia of Chinese Medicine; Chemical Industry Press: Beijing, China; Volume 1, pp. 130–131.
- Zhao, Z.Z.; Liang, Z.T.; Chan, K.; Lu, G.H.; Lee, E.L.M.; Chen, H.B.; Li, L. A unique Issue in the standardization of Chinese Materia Medica: processing. Planta Med. 2010, 76, 1975–1986. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Mao, C. Analgesic and anti-inflammatory effects of different kinds of Corydalis yanhusuo. Shizhen Guoyi Guoyao 2009, 20, 449–450. [Google Scholar]
- Cao, L.; Dou, Z.Y.; Ping, W.; Tian, Y. Effect of different processes on the contents of active ingredients in Corydalis. Chin. J. Inf. Tradit. Chin. Med. 2009, 16, 42–44. [Google Scholar]
- Cheng, X.Y.; Shi, Y.; Zhen, S.L.; Sun, H.; Jin, W. HPLC-MS analysis of ethanol extract of Corydalisyanhusuo and simultaneous determination of eight protoberberine quaternary alkaloids by HPLC-DAD. J. Chromatogr. Sci. 2010, 48, 441–444. [Google Scholar]
- Ma, H.; Wang, Y.; Guo, T.; He, Z.; Chang, X.; Pu, X. Simultaneous determination of tetrahydropalmatine, protopine, and palmatine in rat plasma by LC-ESI-MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2009, 49, 440–446. [Google Scholar] [CrossRef]
- Liu, Y. The Esszential Book Traditional Chinese Medicine; Columbia University Press: New York, NY, USA, 1988; pp. 50–53. [Google Scholar]
- Pharmacopoeia Commission, Pharmacopoeia of Chinese Medicine; Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 446–447.
- Lai, C.K.; Chan, A.Y. Tetrahydropalmatine poisoning: Diagnoses of nine adult overdoses based on toxicology screens by HPLC with diode-array detection and gas chromatography-mass spectrometry. Clin. Chem. 1999, 45, 229–236. [Google Scholar]
- Jiang, X.R.; Wu, Q.X.; Shi, H.L.; Chen, W.P.; Chang, S.Q.; Zhao, S.Y.; Tian, X.Y.; Zhou, L.F.; Guo, S.M.; Li, Y.J. Pharmacological actions of dehydrocorydaline on cardiovascular system. Acta pharmacol. Sin. 1982, 17, 61–65. [Google Scholar]
- Flaw, B.; Sionneau, P. The Treatment of Modern Western Medical Diseases with Chinese Medicine; Blue Poppy Press: Boulder, CO, USA, 2001; pp. 193–196. [Google Scholar]
- Li, B.; Wu, Q.; Shi, J.S.; Sun, A.S.; Huang, X.N. Effects of protopine on intracellular calcium and the PKC activity of rat aorta smooth muscle. Sheng Li Xue Bao 2005, 57, 240–246. [Google Scholar]
- Xiao, X.; Liu, J.; Hu, J.; Zhu, X.; Yang, H.; Wang, C.; Zhang, Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef]
- Ma, Z.J.; Li, X.D.; Gu, X.Z.; Cheng, L.P.; Mao, S.J. Effects of different types and standard of processing vinegaron inherent constituents in rhizoma of Corydalis yanhusuo. Zhongguo Zhong Yao Za Zhi 2006, 31, 465–467. [Google Scholar]
- Sample Availability: Samples of the compounds tetrahydropalmatine, dehydrocorydaline and protopine are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dou, Z.; Li, K.; Wang, P.; Cao, L. Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats. Molecules 2012, 17, 951-970. https://doi.org/10.3390/molecules17010951
Dou Z, Li K, Wang P, Cao L. Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats. Molecules. 2012; 17(1):951-970. https://doi.org/10.3390/molecules17010951
Chicago/Turabian StyleDou, Zhiying, Kefeng Li, Ping Wang, and Liu Cao. 2012. "Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats" Molecules 17, no. 1: 951-970. https://doi.org/10.3390/molecules17010951
APA StyleDou, Z., Li, K., Wang, P., & Cao, L. (2012). Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats. Molecules, 17(1), 951-970. https://doi.org/10.3390/molecules17010951