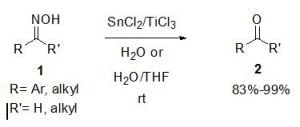
SnCl2/TiCl3-Mediated Deoximation of Oximes in an Aqueous Solvent
Abstract
:1. Introduction
2. Results and Discussion
| Entry | Metal | Time, conversion b |
|---|---|---|
| 1 | TiCl3 | 4 h, 12% |
| 2 | SnCl2 | 4 h, 14% |
| 3 | SnCl2/TiCl3 | 4 h, 99+% |
| 4 | SnCl2/KI | 4 h, 14% |
| 5 | Sn/TiCl3 | 4 h, 80% |
| 6 | Mn/TiCl3 | 4 h, 23% |
| 7 | In/TiCl3 | 4 h, 55% |
| 8 | Fe/TiCl3 | 4 h, 53% |
| 9 | Cu/TiCl3 | 4 h, 39% |
| 10 | Zn/TiCl3 | 4 h, 56% c |
| 11 d | TiCl3 | 4 h, 92% |
| 12 d | SnCl2 | 4 h, 26% |
| Entry | Substrate | Time, yield | Entry | Substrate | Time, yield |
|---|---|---|---|---|---|
| 1 |  | 4 h, 96% | 9 |  | 5 h, 89% |
| 2 |  | 6 h, 92% | 10 |  | 9 h, 97% |
| 3 |  | 9 h, 92% | 11 |  | 6 h, 97% |
| 4 |  | 4 h, 84% | 12 |  | 9 h, 99% |
| 5 |  | 9 h, 82% | 13 |  | 9 h, 83% |
| 6 |  | 9 h, 98% | 14 |  | 3 h, 91% |
| 7 |  | 3 h, 95% | 15 |  | 7 h, 92% |
| 8 |  | 2.5 h, 94% |
| Entry | Substrate | Metal | Solvent | Time | Conversion b (%) |
|---|---|---|---|---|---|
| 1 |  | TiCl3 | H2O | 2 h | 33 |
| 2 | 1p | SnCl2 | H2O | 2 h | 4 |
| 3 | 1p | SnCl2/TiCl3 | H2O | 2 h | 99 (96) c |
| 4 |  | TiCl3 | H2O | 5 h | 12 |
| 5 | 1q | SnCl2 | H2O | 5 h | <1 |
| 6 | 1q | SnCl2/TiCl3 | H2O | 5 h | 99 (93) c |
| 7 |  | TiCl3 | THF/H2O (1/3) | 4 h | 17 |
| 8 | 1r | SnCl2 | THF/H2O (1/3) | 4 h | 9 |
| 9 | 1r | SnCl2/TiCl3 | THF/H2O (1/3) | 4 h | 99 (97) c |
| 10 | 1r | SnCl2/TiCl3 | H2O | 6 h | 71 |
3. Experimental
3.1. General Information and Materials
3.2. General Procedure for SnCl2/TiCl3-Mediated Deoximation of Ketoximes in an Aqueous Solvent
3.3. General Procedure for SnCl2/TiCl3-Mediated Deoximation of Aldoximes in an Aqueous Solvent
4. Conclusions
Supplementary Materials
Acknowledgments
- Samples Availability: Samples of the all compounds are available from the authors.
References and Notes
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 4th ed; John Wiley: New York, NY, USA, 2007; pp. 506–527. [Google Scholar]
- Shriner, R.L.; Fuson, R.C.; Curtin, D.Y.; Morrill, T.C. The Systematic Identification of Organic Compounds, 6th ed; John Wiley: New York, NY, USA, 1980. [Google Scholar]
- Wang, K.; Qian, X.; Cui, J. One step from nitro to oxime: A convenient preparation of unsaturated oximes by the reduction of the corresponding vinylnitro compounds. Tetrahedron 2009, 65, 10377–10382. [Google Scholar] [CrossRef]
- Domingo, L.R.; Picher, M.T.; Arroyo, P.; Sez, J.A. 1,3-Dipolar cycloadditions of electrophilically activated benzonitrile N-oxides. Polar cycloaddition versus oxime formation. J. Org. Chem. 2006, 71, 9319–9330. [Google Scholar]
- Czekelius, C.; Carreira, E.M. Convenient transformation of optically active nitroalkanes into chiral aldoximes and nitriles. Angew. Chem. Int. Ed. 2005, 44, 612–615, and references cited therein. [Google Scholar]
- Suzuki, K.; Watanabe, T.; Murahashi, S.-I. Aerobic oxidation of primary amines to oximes catalyzed by DPPH and WO3/Al2O3. Angew. Chem. Int. Ed. 2008, 47, 2079–2081. [Google Scholar]
- Quan, N.; Shi, X.-X.; Nie, L.-D.; Dong, J.; Zhu, R.-H. A green chemistry method for the regeneration of carbonyl compounds from oximes by using cupric chloride dihydrate as a recoverable promoter for hydrolysis. Synlett 2011, 7, 1028–1032, and references cited therein. [Google Scholar]
- Majireck, M.M.; Witek, J.A.; Weinreb, S.M. An expedient reductive method for conversion of ketoximes to the corresponding carbonyl compounds. Tetrahedron Lett. 2010, 51, 3555–3557. [Google Scholar] [CrossRef]
- Martin, M.; Martinez, G.; Urpi, F.; Vilarrasa, J. Conversion of ketoximes to ketones with trimethylphosphine and 2,2’-dipyridyl diselenide. Tetrahedron Lett. 2004, 45, 5559–5561. [Google Scholar] [CrossRef]
- Lukin, K.A.; Narayanan, B.A. The reaction of oximes with tributylphosphine-phenyldisulfide: Mechanistic insight and new synthetic possibilities. Tetrahedron 2002, 58, 215–219. [Google Scholar] [CrossRef]
- Akazome, M.; Tsuji, Y.; Watanabe, Y. Ruthenium complex-catalyzed selective deoxygenation of ketoximes to ketimines. Chem. Lett. 1990, 635–638. [Google Scholar]
- Curran, D.P.; Brill, J.F.; Rakiewicz, D.M. A mild reductive conversion of oximes to ketones. J. Org.Chem. 1984, 49, 1654–1656. [Google Scholar] [CrossRef]
- Zhou, X.-T.; Yuan, Q.-L.; Ji, H.-B. Highly efficient aerobic oxidation of oximes to carbonyl compounds catalyzed by metalloporphyrins in the presence of benzaldehyde. Tetrahedron Lett. 2010, 51, 613–617. [Google Scholar]
- Shaabani, A.; Farhangi, E. Cobalt(II) phthalocyanine catalyzed aerobic regeneration of carbonyl compounds from the corresponding oximes in 1-butyl-3-methylimidazolium bromide. Appl. Catal. A 2009, 371, 148–152. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Barik, S.K. A facile, catalytic deoximation method using potassium bromide and ammonium heptamolybdate in the presence of hydrogen peroxide in an aqueous medium. Synthesis 2008, 425–428. [Google Scholar] [CrossRef]
- Gupta, P.K.; Manral, L.; Ganesan, K. An efficient approach for the conversion of oximes into carbonyl compounds using dichloramine-T. Synthesis 2007, 1930–1932. [Google Scholar]
- Gogoi, P.; Hazarika, P.; Konwar, D. Surfactant/I2/Water: An efficient system for deprotection of oximes and imines to carbonyls under neutral conditions in water. J. Org. Chem. 2005, 70, 1934–1936. [Google Scholar] [CrossRef]
- Shaabani, A.; Naderi, S.; Rahmati, A.; Badri, Z.; Darvishi, M.; Lee, D.G. Cleavage of oximes, semicarbazones, and phenylhydrazones with supported potassium permanganate. Synthesis 2005, 3023–3025. [Google Scholar]
- Khazaei, A.; Manesh, A.A. Microwave-assisted chemoselective cleavage of oximes to their corresponding carbonyl compounds using 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) as a new deoximating reagent. Synthesis 2005, 1929–1931. [Google Scholar] [CrossRef]
- Chavan, S.P.; Soni, P. A facile deprotection of oximes using glyoxylic acid in an aqueous medium. Tetrahedron Lett. 2004, 45, 3161–3162. [Google Scholar] [CrossRef]
- Maynez, S.R.; Pelavin, L.; Erker, G. Regeneration of ketones from tosylhydrazones, arylhydrazones, and oximes by exchange with acetone. J. Org. Chem. 1975, 40, 3302–3303. [Google Scholar] [CrossRef]
- DePuy, C.H.; Ponder, B.W. Levulinic acid as a reagent for the hydrolysis of oximes and 2,4-dinitrophenylhydrazones. J. Am. Chem. Soc. 1959, 81, 4629–4631. [Google Scholar] [CrossRef]
- Luca, L.D.; Giacomelli, G.; Porcheddu, A. Beckmann rearrangement of oximes under very mild conditions. J. Org. Chem. 2002, 67, 6272–6274. [Google Scholar] [CrossRef]
- Gawley, R.E. The Beckmann reactions: Rearrangements, elimination-additions, fragmentations, and rearrangement-cyclizations. Org. React. 1988, 35, 1–420, and references cited therein. [Google Scholar]
- Pereyre, M.; Quintard, J.-P.; Rahm, A. Tin in Organic Synthesis; Butterworth: London, UK, 1987. [Google Scholar]
- Smith, P.J. Toxicological Data on Organotin Compounds; International Tin Research Inst.: London, UK, 1978. [Google Scholar]
- Das, N.B.; Nayak, A.; Nanda, B. A general approach for the microwave-assisted regeneration of carbonyl compounds from their nitrogenous derivatives. J. Chem. Res. 2004, 712–713. [Google Scholar]
- Das, N.B.; Nanda, B.; Nayak, A. SnCl2-SiO2: A selective reagent for efficient regeneration of carbonyls from nitrogenous derivatives. Synth. Commun. 2002, 32, 3647–3651. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hung, S.-F.; Lin, L.-Z.; Tsai, W.-S.; Chuang, T.-H. Tin mediated allylation reactions of enol ethers in water. Org. Lett. 2011, 13, 332–335. [Google Scholar] [CrossRef]
- Gremyachinskiy, D.E.; Smith, L.L.; Gross, P.H.; Samoshin, V.V. Facile synthesis of homoallylic alcohols from aldehyde acetals in water. Green Chem. 2002, 4, 317–318. [Google Scholar]
- Samoshin, V.V.; Gremyachinskiy, D.E.; Smith, L.L.; Bliznets, I.V.; Gross, P.H. Practical synthesis of bis-homoallylic alcohols from dialdehydes or their acetals. Tetrahedron Lett. 2002, 43, 6329–6330. [Google Scholar] [CrossRef]
- Juan, S.; Hua, Z.-H.; Qi, S.; Ji, S.-J.; Loh, T.-P. Indium trichloride-catalyzed indium-mediated allylation of dihydropyrans and dihydrofurans in water. Synlett 2004, 5, 829–830. [Google Scholar]
- Due to poor solubility of ketoximes in water, THF is added to increase the reaction rate. Based on screening results, amounts of SnCl2 and TiCl3 (1.5 equivalents each) are chosen as the general quantity for deoximation of various ketoximes.
- Houllemare, D.; Outurquin, F.; Paulmier, C. Synthesis of homoallylic (but-3-enylic) alcohols from aldehydes with allylic chlorides, tin(II) chloride and potassium iodide in water. J. Chem. Soc. Perkin Trans. 1 1997, 1629–1632. [Google Scholar]
- Lee, S.Y.; Lee, B.S.; Lee, C.-W.; Oh, D.Y. Synthesis of 4-oxo-2-alkenylphosphonates via nitrile oxide cycloaddition: γ-Acylation of allylic phosphonates. J. Org. Chem. 2000, 65, 256–257. [Google Scholar] [CrossRef]
- Timms, G.H.; Wildsmith, E. Reduction of oximes with tervalent titanium, a mild deoximation procedure and the partial synthesis of erythromycylamine. Tetrahedron Lett. 1971, 2, 195–198. [Google Scholar]
- Balicki, R.; Kaczmarek, Ł.; Malinowski, M. Facile reductive cleavage of oximes with a low-valent titanium reagent. Liebigs Ann. Chem. 1989, 1139–1140. [Google Scholar]
- McMurry, J.E. Organic chemistry of low-valent titanium. Acc. Chem. Res. 1974, 7, 281–286. [Google Scholar] [CrossRef]
- Ramón, R.S.; Bosson, J.; Díez-González, S.; Marion, N.; Nolan, S.P. Au/Ag-cocatalyzed aldoximes to amides rearrangement under solvent- and acid-free conditions. J. Org. Chem. 2010, 75, 1197–1202. [Google Scholar]
- Nobuta, T.; Hirashima, S.-I.; Tada, N.; Miura, T.; Itoh, A. One-pot metal-free syntheses of acetophenones from styrenes through aerobic photo-oxidation and deiodination with iodine. Org. Lett. 2011, 13, 2576–2579. [Google Scholar] [CrossRef]
- Bonvin, Y.; Callens, E.; Larrosa, I.; Henderson, D.A.; Oldham, J.; Burton, A.J.; Barrett, A.G.M. Bismuth-catalyzed benzylic oxidations with tert-butyl hydroperoxide. Org. Lett. 2005, 7, 4549–4552. [Google Scholar] [CrossRef]
- Ushkov, A.V.; Grushin, V.V. Rational catalysis design on the basis of mechanistic understanding: Highly efficient Pd-catalyzed cyanation of aryl bromides with NaCN in recyclable solvents. J. Am. Chem. Soc. 2011, 133, 10999–11005. [Google Scholar] [CrossRef]
- Ruan, J.; Iggo, J.A.; Berry, N.G.; Xiao, J. Hydrogen-bonding-promoted oxidative addition and regioselective arylation of olefins with aryl chlorides. J. Am. Chem. Soc. 2010, 132, 16689–16699. [Google Scholar]
- Sarvari, H.M.; Sharghi, H. Zinc oxide (ZnO) as a new, highly efficient, and reusable catalyst for acylation of alcohols, phenols and amines under solvent free conditions. Tetrahedron 2005, 61, 10903–10907. [Google Scholar] [CrossRef]
- Zhang, G.; Wen, X.; Wang, Y.; Mo, W.; Ding, C. Sodium nitrite catalyzed aerobic oxidative deoximation under mild conditions. J. Org. Chem. 2011, 76, 4665–4668. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, B.; Deng, C.-L.; Tang, R.-Y.; Zhang, X.-G.; Li, J.-H. Palladium-catalyzed oxidative coupling of trialkylamines with aryl iodides leading to alkyl aryl ketones. Org. Lett. 2011, 13, 2184–2187. [Google Scholar] [CrossRef]
- Zhu, F.-X.; Wang, W.; Li, H.-X. Water-medium and solvent-free organic reactions over a bifunctional catalyst with Au nanoparticles covalently bonded to HS/SO3H functionalized periodic mesoporous organosilica. J. Am. Chem. Soc. 2011, 133, 11632–11640. [Google Scholar]
- Huffman, J.W. A new synthetic route to methoxytetralones. J. Org. Chem. 1959, 24, 1759–1763. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, C.; He, X.; Ju, K.; Zhang, M.; Yu, S.; Wu, J. DMAP-catalyzed cascade reaction: one-pot synthesis of benzofurans in water. Tetrahedron 2010, 66, 9629–9633. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Yoshida, T.; Imori, Y.; Yamaguchi, R. Dehydrogenative oxidation of primary and secondary alcohols catalyzed by a Cp*Ir complex having a functional C,N-chelate ligand. Org. Lett. 2011, 9, 2278–2281. [Google Scholar]
- Schultz, M.J.; Hamilton, S.S.; Jensen, D.R.; Sigman, M.S. Development and comparison of the substrate scope of Pd-catalysts for the aerobic oxidation of alcohols. J. Org. Chem. 2005, 70, 3343–3352. [Google Scholar]
- Shen, Z.; Dai, J.; Xiong, J.; He, X.; Mo, W.; Hu, B.; Sun, N.; Hu, X. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)/tert-butyl nitrite/oxygen: A versatile catalytic oxidation system. Adv. Synth. Catal. 2011, 353, 3031–3038. [Google Scholar] [CrossRef]
- Kompis, I.; Wick, A. Synthesis of 4-halo-substituted analogs of trimethoprim. Helv. Chim. Acta 1977, 60, 3025–3034. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, M.-H.; Liu, H.-J.; Chang, C.-Y.; Lin, W.-C.; Chuang, T.-H. SnCl2/TiCl3-Mediated Deoximation of Oximes in an Aqueous Solvent. Molecules 2012, 17, 2464-2473. https://doi.org/10.3390/molecules17032464
Lin M-H, Liu H-J, Chang C-Y, Lin W-C, Chuang T-H. SnCl2/TiCl3-Mediated Deoximation of Oximes in an Aqueous Solvent. Molecules. 2012; 17(3):2464-2473. https://doi.org/10.3390/molecules17032464
Chicago/Turabian StyleLin, Mei-Huey, Han-Jun Liu, Cheng-Yu Chang, Wei-Cheng Lin, and Tsung-Hsun Chuang. 2012. "SnCl2/TiCl3-Mediated Deoximation of Oximes in an Aqueous Solvent" Molecules 17, no. 3: 2464-2473. https://doi.org/10.3390/molecules17032464
APA StyleLin, M. -H., Liu, H. -J., Chang, C. -Y., Lin, W. -C., & Chuang, T. -H. (2012). SnCl2/TiCl3-Mediated Deoximation of Oximes in an Aqueous Solvent. Molecules, 17(3), 2464-2473. https://doi.org/10.3390/molecules17032464