Hypervalent Nonbonded Interactions of a Divalent Sulfur Atom. Implications in Protein Architecture and the Functions
Abstract
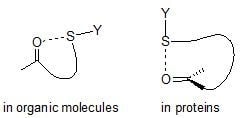
:1. Introduction


2. S···X Interactions in Organic Compounds
2.1. Database Analysis
2.2. Energetic Elements of S···X interactions
2.3. Examples

3. S···X Interactions in Proteins
3.1. Database Analysis
| X = | d b ≤ 0.0 Ǻ | 0.0 < d ≤ 0.5 Ǻ |
|---|---|---|
| O | 100 | 664 |
| N | 33 | 359 |
| S | 15 | 68 |
| C | 134 | 1478 |
| Others | 2 | 0 |
| Total | 284 | 2569 |
3.2. Energetic Elements of S···X Interactions


3.3. Examples
4. Implications of S···X Interactions in Protein Architecture


5. Implications of S···X Interactions in Protein Functions
5.1. Phospholipase A2 (PLA2) [20]

5.2. Ribonuclease A (RNase A)

5.3. Insulin

5.4. Lysozyme

6. Method of Database Analysis
7. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- Ciuffarin, E.; Guaraldi, G. Nucleophilic substitution at dicoordinated sulfur. Effect of the leaving group on the reaction between triphenylmethyl sulfenyl derivatives and n-butylamine. J. Org. Chem. 1970, 35, 2006–2010. [Google Scholar]
- Rosenfield, R.E., Jr.; Parthasarathy, R.; Dunitz, J.D. Directional preferences of nonbonded atomic contacts with divalent sulfur. 1. Electrophiles and nucleophiles. J. Am. Chem. Soc. 1977, 99, 4860–4862. [Google Scholar] [CrossRef]
- Row, T.N.G.; Parthasarathy, R. Directional preferences of nonbonded atomic contacts with divalent sulfur in terms of its orbital orientations. 2. S···S interactions and nonspherical shape of sulfur in crystals. J. Am. Chem. Soc. 1981, 103, 477–479. [Google Scholar]
- Desiraju, G.R.; Nalini, V. Database analysis of crystal-structure-determining interactions involving sulphur: Implications for the design of organic metals. J. Mater. Chem. 1991, 1, 201–203. [Google Scholar] [CrossRef]
- Turbiez, M.; Frère, P.; Allain, M.; Videlot, C.; Ackermann, J.; Roncali, J. Design of organic semiconductors: Tuning the electronic properties of π-conjugated oligothiophenes with the 3,4-ethylenedioxythiophene (EDOT) building block. Chem. Eur. J. 2005, 11, 3742–3752. [Google Scholar] [CrossRef]
- Réthoré, C.; Madalan, A.; Fourmigué, M.; Canadell, E.; Lopes, E.B.; Almeida, M.; Clérac, R.; Avarvari, N. O··· S vs. N···S intramolecular nonbonded interactions in neutral and radical cation salts of TTF-oxazoline derivatives: synthesis, theoretical investigations, crystalline structures, and physical properties. New J. Chem. 2007, 31, 1468–1483. [Google Scholar]
- Burling, F.T.; Goldstein, B.M. Computational studies of bonbonded sulfur-oxygen and selenium-oxygen interactions in the thiazole and selenazole nucleosides. J. Am. Chem. Soc. 1992, 114, 2313–2320. [Google Scholar] [CrossRef]
- Nagao, Y.; Hirata, T.; Goto, S.; Sano, S.; Kakehi, A.; Iizuka, K.; Shiro, M. Intramolecular nonbonded S···O interaction recognized in (acylimino)thiadiazoline derivatives as angiotensin II receptor antagonists and related compounds. J. Am. Chem. Soc. 1998, 120, 3104–3110. [Google Scholar]
- Nakanishi, W.; Nakamoto, T.; Hayashi, S.; Sasamori, T.; Tokitoh, N. Atoms-in-molecules analysis of extended hypervalent five-center, six-electron (5c-6e) C2Z2O interactions at the 1,8,9-positions of anthraquinone and 9-methoxyanthracene systems. Chem. Eur. J. 2007, 13, 255–268. [Google Scholar]
- Bleiholder, C.; Werz, D.B.; Köppel, H.; Gleiter, R. Theoretical investigations on chalcogen-chalcogen interactions: What makes these nonbonded interactions bonding? J. Am. Chem. Soc. 2006, 128, 2666–2674. [Google Scholar]
- Dahaoui, S.; Pichon-Pesme, V.; Howard, J.A.K.; Lecomte, C. CCD charge density study on crystals with large unit cell parameters: The case of hexagonal L-cystine. J. Phys. Chem. A 1999, 103, 6240–6250. [Google Scholar]
- Nauser, T.; Jacoby, M.; Koppenol, W.H.; Squier, T.C.; Schöneich, C. Calmodulin methionine residues are targets for one-electron oxidation by hydroxyl radicals: Formation of S.˙.N three-electron bonded radical complexes. Chem. Commun. 2005, 587–589. [Google Scholar]
- Mishra, B.; Sharma, A.; Naumov, S.; Priyadarsini, K.I. Novel reactions of one-electron oxidized radicals of selenomethionine in comparison with methionine. J. Phys. Chem. B 2009, 113, 7709–7715. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.Z. The bifurcate chalcogen bond: Some theoretical observations. J. Mol. Struct. (Theochem) 2009, 916, 135–138. [Google Scholar]
- Zhao, Q.; Feng, D.C.; Sun, Y.M.; Hao, J.C.; Cai, Z.T. Theoretical investigations on the weak nonbonded C=S···CH2 interactions: Chalcogen-bonded complexes with singlet carbene as an electron donor. Int. J. Quantum Chem. 2011, 111, 3881–3887. [Google Scholar]
- Pal, D.; Chakrabarti, P. Non-hydrogen bond interactions involving the methionine sulfur atom. J. Biomol. Struct. Dynam. 2001, 19, 115–128. [Google Scholar] [CrossRef]
- Iwaoka, M.; Takemoto, S.; Okada, M; Tomoda, S. Statistical characterization of nonbonded S···O interactions in proteins. Chem. Lett. 2011, 132–133. [Google Scholar]
- Iwaoka, M.; Takemoto, S.; Okada, M.; Tomoda, S. Weak nonbonded S···X (X=O, N, and S) interactions in proteins. Statistical and theoretical studies. Bull. Chem. Soc. Jpn. 2002, 75, 1611–1625. [Google Scholar] [CrossRef]
- Iwaoka, M.; Takemoto, S.; Tomoda, S. Statistical and theoretical investigations on the directionality of nonbonded S···O interactions. Implications for molecular design and protein engineering. J. Am. Chem. Soc. 2002, 124, 10613–10620. [Google Scholar]
- Iwaoka, M.; Isozumi, N. Possible roles of S···O and S···N interactions in the functions and evolution of phospholipase A2. Biophysics 2006, 2, 23–34. [Google Scholar] [CrossRef]
- Carugo, O. Stereochemistry of the interaction between methionine sulfur and the protein core. Biol. Chem. 1999, 380, 495–498. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Pal, D.; Chakrabarti, P. Disulfide bonds, their stereospecific environment and conservation in protein structures. Protein Eng. Des. Sel. 2004, 17, 795–808. [Google Scholar] [CrossRef]
- Lu, J.M.; Lu, Y.X.; Yang, S.B.; Zhu, W.L. Theoretical and crystallographic data investigations of noncovalent S···O interactions. Struct. Chem. 2011, 22, 757–763. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Simultaneous σ-hole and hydrogen bonding by sulfur- and selenium-containing heterocycles. Int. J. Quantum Chem. 2008, 108, 2770–2781. [Google Scholar] [CrossRef]
- Wang, W.Z.; Ji, B.M.; Zhang, Y. Chalcogen bond: A sister noncovalent bond to halogen bond. J. Phys. Chem. A 2008, 113, 8132–8135. [Google Scholar]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. 2003, B58, 380–388. [Google Scholar]
- Kucsman, A.; Kapovits, I. Nonbonded Sulfur-Oxygen Interaction in Organic Sulfur Compounds. In Organic Sulfur Chemistry; Bernardi, F., Csizmadia, I.G., Mangini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; pp. 191–245. [Google Scholar]
- Zauhar, R.J.; Colbert, C.L.; Morgan, R.S.; Welsh, W.J. Evidence for a strong sulfur-aromatic interaction derived from crystallographic data. Biopolymers 2000, 53, 233–248. [Google Scholar] [CrossRef]
- Cozzolino, A.F.; Vargas-Baca, I.; Mansour, S.; Mahmoudkhani, A.H. The nature of the supramolecular association of 1,2,5-chalcogenadiazoles. J. Am. Chem. Soc. 2005, 127, 3184–3190. [Google Scholar]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Atoms-in-molecules dual parameter analysis of weak to strong interactions: Behaviors of electronic energy densitiesversus Laplacian of electron densities at bond critical points. J. Phys. Chem. A 2008, 112, 13593–13599. [Google Scholar] [CrossRef]
- Scheiner, S. On the properties of X···N noncovalent interactions for first-, second-, and third-row X atom. J. Chem. Phys. 2011, 134, 164313. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. The S···N noncovalent interaction: Comparison with hydrogen and halogen bonds. Chem. Phys. Lett. 2011, 514, 36–39. [Google Scholar] [CrossRef]
- Nagao, Y.; Honjo, T.; Iimori, H.; Goto, S.; Sano, S.; Shiro, M.; Yamaguchi, K.; Sei, Y. Intramolecular nonbonded S···O interaction in acetazolamide and thiadiazolinethione molecules in their dimeric crystalline structures and complex crystalline structures with enzymes. Tetrahedron Lett. 2004, 45, 8757–8761. [Google Scholar] [CrossRef]
- Esparza-Ruiz, A.; Peña-Hueso, A.; Hernández-Díaz, J.; Flores-Parra, A.; Contreras, R. Effect of weak sulfur interactions and hydrogen bonds in the folded or unfolded conformation of bis 2-(1H-benzimidazol-2-yl)phenyl disulfide derivatives. Cryst. Growth Des. 2007, 7, 2031–2040. [Google Scholar] [CrossRef]
- González, F.V.; Jain, A.; Rodríguez, S.; Sáez, J.A.; Vicent, C.; Peris, G. Stereoisomerization of β-hydroxy-α-sulfenyl-γ-butyrolactones controlled by two concomitant 1,4-Type nonbonded sulfur–oxygen interactions as analyzed by X-ray crystallography. J. Org. Chem. 2010, 75, 5888–5894. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Omelchenko, I.V.; Kalyuzhny, A.L.; Paponov, B.V. Intramolecular S···O chalcogen bond in thioindirubin. Struct. Chem. 2010, 21, 1005–1011. [Google Scholar] [CrossRef]
- Dobson, C.M.; Sali, A.; Karplus, M. Protein folding: A perspective from theory and experiment. Angew. Chem. Int. Ed. Engl. 1998, 37, 869–893. [Google Scholar]
- Derewenda, Z.S.; Lee, L.; Derewenda, U. The occurrence of C-H···O hydrogen bonds in proteins. J. Mol. Biol. 1995, 252, 248–262. [Google Scholar] [CrossRef]
- Bella, J.; Berman, H.M. Crystallographic evidence for Cα-H···O=C hydrogen bonds in a collagen triple helix. J. Mol. Biol. 1996, 264, 734–742. [Google Scholar] [CrossRef]
- Vargas, R.; Garza, J.; Dixon, D.A.; Hay, B.P. How strong is the Cα–H···O=C hydrogen bond? J. Am. Chem. Soc. 2000, 122, 4750–4755. [Google Scholar]
- Flocco, M.M.; Mowbray, S.L. Planar stacking interactions of arginine and aromatic side-chains in proteins. J. Mol. Biol. 1994, 235, 709–717. [Google Scholar] [CrossRef]
- Gallivan, J.P.; Dougherty, D.A. Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar]
- Gallivan, J.P.; Dougherty, D.A. A computational study of cation-π interactions vs salt bridges in aqueous media: Implications for protein engineering. J. Am. Chem. Soc. 2000, 122, 870–874. [Google Scholar] [CrossRef]
- Umezawa, Y.; Nishio, M. CH/π interactions as demonstrated in the crystal structure of guanine-nucleotide binding proteins, Src homology-2 domains and human growth hormone in complex with their specific ligands. Bioorg. Med. Chem. 1998, 6, 493–504. [Google Scholar] [CrossRef]
- Takahashi, O.; Kohno, Y.; Nishio, M. Relevance of weak hydrogen bonds in the conformation of organic compounds and bioconjugates: Evidence from recent experimental data and high-level ab initio MO calculations. Chem. Rev. 2010, 110, 6049–6076. [Google Scholar] [CrossRef]
- Brandl, M.; Weiss, M.S.; Jabs, A.; Sühnel, J.; Hilgenfeld, R. C–H···π-interactions in proteins. J. Mol. Biol. 2001, 307, 357–377. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Bhattacharyya, R. Geometry of nonbonded interactions involving planar groups in proteins. Prog. Biophys. Mol. Biol. 2007, 95, 83–137. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Alexander, R.S.; Christianson, D.W. Hydrogen bond stereochemistry in protein structure and function. J. Mol. Biol. 1990, 215, 457–471. [Google Scholar] [CrossRef]
- Morgan, R.S.; Tatsch, C.E.; Gushard, R.H.; McAdon, J.M.; Warme, P.K. Chains of alternating sulfur and π-bonded atoms in eight small proteins. Int. J. Peptide Protein Res. 1978, 11, 209–217. [Google Scholar]
- Reid, K.S.C.; Lindley, P.F.; Thornton, J.M. Sulfur-aromatic interactions in proteins. FEBS Lett. 1985, 190, 209–213. [Google Scholar] [CrossRef]
- Viguera, A.R.; Serrano, L. Side-chain interactions between sulfur-containing amino acids and phenylalanine in α-helices. Biochemistry 1995, 34, 8771–8779. [Google Scholar] [CrossRef]
- Cheney, B.V.; Schulz, M.W.; Cheney, J. Complexes of benzene with formamide and methanethiol as models for interactions of protein substructures. Biochim. Biophys. Acta 1989, 996, 116–124. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucl. Acids Res. 2000, 28, 235–242. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on the approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Taylor, J.C.; Markham, G.D. The bifunctional active site of S-adenosylmethionine synthetase—Roles of the active site aspartates. J. Biol. Chem. 1999, 274, 32909–32914. [Google Scholar] [CrossRef]
- Brandt, W.; Golbraikh, A.; Täger, M.; Lendeckel, U. A molecular mechanism for the cleavage of a disulfide bond as the primary function of agonist binding to G-protein-coupled receptors based on theoretical calculations supported by experiments. Eur. J. Biochem. 1999, 261, 89–97. [Google Scholar] [CrossRef]
- Palenchar, J.B.; Crocco, J.M.; Colman, R.F. The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human adenylosuccinate lyase deficiencies. Protein Sci. 2003, 12, 1694–1705. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, N.; Sharma, S.; Shin, K.; Takase, M.; Kaur, P.; Srinivasan, A.; Singh, T.P. Inhibition of lactoperoxidase by its own catalytic product: Crystal structure of the hypothiocyanate-inhibited bovine lactoperoxidase at 2.3-Ǻ resolution. Biophys. J. 2009, 96, 646–654. [Google Scholar]
- Nakamura, T.; Yamamoto, T.; Abe, M.; Matsumura, H.; Hagihara, Y.; Goto, T.; Yamaguchi, T.; Inoue, T. Oxidation of archaeal peroxiredoxin involves a hypervalent sulfur intermediate. Proc. Nat. Acad. Sci. USA 2008, 105, 6238–6242. [Google Scholar]
- Iwaoka, M.; Tomoda, S. The SAAP force field. A simple approach to a new all-atom protein force field by using single amino acid potential (SAAP) functions in various solvents. J. Comput. Chem. 2003, 24, 1192–1200. [Google Scholar]
- Iwaoka, M.; Kimura, N.; Yosida, D.; Minezaki, T. The SAAP force field: Development of the single amino acid potentials for 20 proteinogenic amino acids and Monte Carlo molecular simulation for short peptides. J. Comput. Chem. 2009, 30, 2039–2055. [Google Scholar] [CrossRef]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar]
- Jain, M.K.; Gelb, M.H.; Rogers, J.; Berg, O.G. Kinetic basis for interfacial catalysis by phospholipase A2. Methods Enzymol. 1995, 249, 567–614. [Google Scholar] [CrossRef]
- Yu, B.Z.; Pan, Y.H.; Janssen, M.J.W.; Bahnson, B.J.; Jain, M.K. Kinetic and structural properties of disulfide engineered phospholipase A2: Insight into the role of disulfide bonding patterns. Biochemistry 2005, 44, 3369–3379. [Google Scholar] [CrossRef]
- Ohno, M.; Chijiwa, T.; Oda-Ueda, N.; Ogawa, T.; Hattori, S. Molecular evolution of myotoxic phospholipase A2 from snake venom. Toxicon 2003, 42, 841–854. [Google Scholar] [CrossRef]
- Raines, R.T. Ribonuclease A. Chem. Rev. 1998, 98, 1045–1065. [Google Scholar] [CrossRef]
- Rothwarf, D.M.; Li, Y.J.; Scheraga, H.A. Regeneration of bovine pancreatic ribonuclease A: Identification of two nativelike three-disulfide intermediates involved in separate pathways. Biochemistry 1998, 37, 3760–3766. [Google Scholar] [CrossRef]
- Welker, E.; Narayan, M.; Wedemeyer, W.J.; Scheraga, H.A. Structural determinants of oxidative folding in proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 2312–2316. [Google Scholar] [CrossRef]
- Xu, X.; Rothwarf, D.M.; Scheraga, H.A. Nonrandom distribution of the one-disulfide intermediates in the regeneration of ribonuclease A. Biochemistry 1996, 35, 6406–6417. [Google Scholar] [CrossRef]
- Li, Y.J.; Rothwarf, D.M.; Scheraga, H.A. Mechanism of reductive protein unfolding. Nat. Struct. Mol. Biol. 1995, 2, 489–494. [Google Scholar] [CrossRef]
- Nachman, J.; Miller, M.; Gilliland, G.L.; Carty, R.; Pincus, M.; Wlodawer, A. Crystal structure of two covalent nucleoside derivatives of ribonuclease A. Biochemistry 1990, 29, 928–937. [Google Scholar]
- Leonidas, D.D.; Shapiro, R.; Irons, L.I.; Russo, N.; Acharya, K.R. Crystal structures of ribonuclease A complexes with 5'-diphosphoadenosine 3'-phosphate and 5'-diphosphoadenosine 2'-phosphate at 1.7 Ǻ resolution. Biochemistry 1997, 36, 5578–5588. [Google Scholar]
- Leonidas, D.D.; Shapiro, R.; Irons, L.I.; Russo, N.; Acharya, K.R. Toward rational design of ribonuclease inhibitors: High-resolution crystal structure of a ribonuclease A complex with a potent 3',5'-pyrophosphate-linked dinucleotide inhibitor. Biochemistry 1999, 38, 10287–10297. [Google Scholar]
- de Meyts, P. Insulin and its receptor: Structure, function and evolution. BioEssays 2004, 26, 1351–1362. [Google Scholar] [CrossRef]
- Merlini, G.; Bellotti, V. Lysozyme: A paradigmatic molecule for the investigation of protein structure, function and misfolding. Clin. Chim. Acta 2005, 357, 168–172. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iwaoka, M.; Isozumi, N. Hypervalent Nonbonded Interactions of a Divalent Sulfur Atom. Implications in Protein Architecture and the Functions. Molecules 2012, 17, 7266-7283. https://doi.org/10.3390/molecules17067266
Iwaoka M, Isozumi N. Hypervalent Nonbonded Interactions of a Divalent Sulfur Atom. Implications in Protein Architecture and the Functions. Molecules. 2012; 17(6):7266-7283. https://doi.org/10.3390/molecules17067266
Chicago/Turabian StyleIwaoka, Michio, and Noriyoshi Isozumi. 2012. "Hypervalent Nonbonded Interactions of a Divalent Sulfur Atom. Implications in Protein Architecture and the Functions" Molecules 17, no. 6: 7266-7283. https://doi.org/10.3390/molecules17067266
APA StyleIwaoka, M., & Isozumi, N. (2012). Hypervalent Nonbonded Interactions of a Divalent Sulfur Atom. Implications in Protein Architecture and the Functions. Molecules, 17(6), 7266-7283. https://doi.org/10.3390/molecules17067266