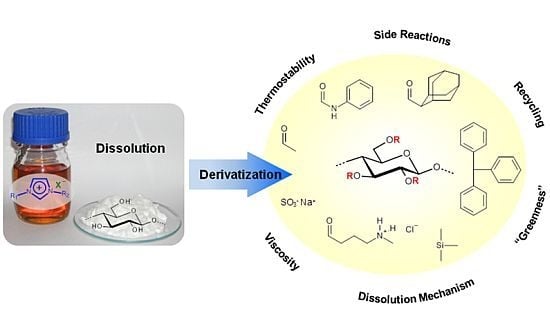
Ionic Liquids — Promising but Challenging Solvents for Homogeneous Derivatization of Cellulose
Abstract
:1. Introduction

2. History
2.1. Dissolution of Cellulose in Molten Organic Salts

2.2. Ionic Liquids as Reaction Media for Cellulose Derivatization
| Entry | Substituent (Cell-OR) | DS a | ILs b | Comments c | Ref. |
|---|---|---|---|---|---|
| carbamates | |||||
| 1 |  phenyl carbamate phenyl carbamate | 0.5–3.0 | BMIMCl | [36] | |
| 0.3–3.0 | BMIMCl | - derivatization of bacterial cellulose | [37] | ||
| 2 |  3-(triethoxysilyl)propyl carbamete 3-(triethoxysilyl)propyl carbamete | 0.43, 2.95 | BMIMCl | - hydrolysis of ethoxysilyl groups directly after synthesis | [38] |
| carboxylic acid esters | |||||
| 3 |  acetate acetate | 0.9–2.8 | AMIMCl | [39,40] | |
| 1.9–3.0 | BMIMCl, EMIMCl, BDMIMCl, ADMIMBr | [36,41] | |||
| 0.7–3.0 | BMIMCl | - derivatization of bacterial cellulose | [37] | ||
| 4 |  propionate/butyrate/… and mixed esters propionate/butyrate/… and mixed esters | ≈0.5–2.9 | ABIMCl, BMIMAc, DOHMIMAc, HMIMAc, MOEMIMAc | - cellulose esters and mixed esters prepared- microwave irradiation (partly)- also pentanoates and hexanoates prepared | [42,43,44] |
| 1.4–2.7(DSoverall) | AMIMCl | - acetate-butyrates and acetate-propionates prepared- derivatization of cellulose from sugarcane bagasse | [45] | ||
| 0.5–2.9(DSoverall) | BMIMAc, BMIMCl, BMIMDMP, BMIMPr, EMIMAc, TBMADMP | - cellulose esters and mixed esters prepared- co-solvents applied (partly)- also mixed esters with benzoate prepared | [16,17,18,19,20] | ||
| 0.2–2.5 | BMIMCl, EMIMAc | - heterogeneous conversion with gaseous ketenes- acetates, propionates, and pentanoates prepared | [21] | ||
| 5 |  benzoates benzoates | 1.0–3.0 | AMIMCl | [46] | |
| 6 |  fuorate fuorate | 0.5–3.0 | BMIMCl | - activation of carboxylic acid with N,N’-carbonyldiimidazole (partly) | [47] |
| 7 |  laurate laurate | 0.3–1.5 | BMIMCl | - phase separation with increasing DS | [36] |
| 8 |  stearate stearate | 2.2–2.6 | BMIMCl | [48] | |
| 9 |  pivalate, adamantate, trimethylbenzoate pivalate, adamantate, trimethylbenzoate | 0.9–1.4 | AMIMCl | - comparison with DMA/LiCl and DMSO/TBAF | [49] |
| 10 |  succinate, phthalate succinate, phthalate | 0.2–2.5 | AMIMCl, BMIMCl | - derivatization of cellulose from sugarcane bagasse- catalysts applied - co-solvents utilized (partly) | [50,51,52,53] |
| 11 |  glutarate glutarate | 0.3–1.2 | BMIMCl | - ultrasound irradiation | [54] |
| 12 |  carboxylates containing amino groups carboxylates containing amino groups | 0.1-1.2 | BMIMCl | - tosyl chloride used to form reactive intermediates- co-solvents utilized | [55,56] |
| 13 |  oxy-carboxylic acid esters oxy-carboxylic acid esters | 0.1–3.0 | AMIMCl, BMIMCl, EMIMCl | - activation of oxy-carbonic acid with N,N’-carbonyldiimidazole- derivatization of bacterial cellulose (partly) | [57] |
| 14 |  2-halo carboxylate macro-initiators 2-halo carboxylate macro-initiators | 0.6–1.0 | AMIMCl | - co-solvents utilized- bromo compounds utilized | [58,59,60] |
| 0.3–1.9 | BMIMCl | - chloro compounds utilized | [61] | ||
| 15 |  dehydroabiatate dehydroabiatate | 1.4–1.9 | BMIMBr | - catalyst applied | [62] |
| 16 |  pyro-pheophorbide pyro-pheophorbide | 0.07 | AMIMCl | - activation of acid with N,N'-carbonyldiimidazole | [63] |
| ethers | |||||
| 17 |  hydroxethyl/hydroxypropyl hydroxethyl/hydroxypropyl | 0.1–2.2 | BMIMCl, BDMIMCl, BDTAC, EMIMAc | - co-solvents utilized | [64,65] |
| 18 |  trityl/methoxytrityl trityl/methoxytrityl | 0.8, 1.8 | AMIMCl | - pyridine utilized as base and co-solvent | [66] |
| 0.8–1.4 | BMIMCl | - pyridine utilized as base and co-solvent | [67] | ||
| 19 |  carboxymethyl carboxymethyl | 0.49 | BMIMCl | - co-solvents utilized- heterogeneous (solid NaOH used as base)- gel-like system formed | [36] |
| n.a. | BMIMCl | - heterogeneous (solid NaOH used as base) | [68] | ||
| 20 |  trimethylsilyl trimethylsilyl | 0.4–2.9 | BMIMCl, EMIMAc | - co-solvents utilized | [69] |
| 0.2–3.0 | BMIMCl, BMIMAc, BMIMBz, BMIMPr, EMIMAc, EMIMDEP | - heterogeneous derivatization (polar and non-polar liquid phase) | [70] | ||
| sulfuric/sulfonic acid esters | |||||
| 21 |  sulfate sulfate | 0.1–1.5 | AMIMCl, BMIMCl, EMIMCl | - co-solvents utilized | [71,72] |
| 1.3–1.7 | BMIMCl | - co-solvents utilized | [73] | ||
| 22 |  tosylate tosylate | 0.1–1.1 | AMIMCl, BMIMCl | - co-solvents utilized, reaction at 25 °C | [74] |
| 0.84 | AMIMCl | - reaction at 10 °C | [63] | ||
| deoxy cellulose derivatives | |||||
| 23 |  chloro-deoxy chloro-deoxy | 0.8–1.l | BMIMCl | - co-solvent utilized- strong polymer degradation | [75] |
| grafts | |||||
| 24 |  graft-poly(L-lactide) graft-poly(L-lactide) | 0.7–2.7(1.4–4.5) d | AMIMCl | - grafting by ring-opening of L-lactide- 4-dimethylamino pyridine applied as catalyst | [76] |
| 25 |  graft-poly(acrylic acid) graft-poly(acrylic acid) | n.a. | BMIMCl | - initiation by persulfate - microwave irradiation | [77] |
| 26 |  graft-poly(N-isopropylacrylamide) graft-poly(N-isopropylacrylamide) | n.a. | BMIMCl | - initiation by γ-ray irradiation | [78] |
| 27 |  graft-poly(methyl methacrylate) graft-poly(methyl methacrylate) | 0.3–1.9 | BMIMCl | - derived from 14 by atom transfer radical polymerization | [61] |
2.2.1. Esterification
2.2.2. Etherification
2.2.3. Other Reactions
3. Difficulties and Drawbacks
3.1. Purity of Ionic Liquids
3.2. Viscosity: A Matter of Kinetics vs. Thermodynamics
| Ionic Liquid a | Temperature, | Viscosity, | Ref. | ||
|---|---|---|---|---|---|
| Cation | Anion | °C | mPa·s | ||
| AMIM+ | Cl− | 50 | 120 | [112] | |
| AMIM+ | Fo− | 25 | 66 | [113] | |
| BMIM+ | Ac− | 25 | 485 | [114] | |
| BMIM+ | Ac− | 80 | 26 | [114] | |
| BMIM+ | Ac− | 100 | 15 | [114] | |
| BMIM+ | Ac− | 120 | 9 | [114] | |
| BMIM+ | Cl− | 80 | 142 | [114] | |
| BMIM+ | Cl− | 100 | 68 | [114] | |
| BMIM+ | Cl− | 120 | 31 | [114] | |
| BMIM+ | Fo− | 25 | 38 | [112] | |
| BMIM+ | DMP− | 20 | 696 | [115] | |
| BMIM+ | DMP− | 80 | ≈30 | [115] | |
| EMIM+ | Ac− | 21 | 180 | [116] | |
| EMIM+ | Ac− | 25 | 162 | [114] | |
| EMIM+ | Ac− | 80 | 17 | [114] | |
| EMIM+ | Ac− | 100 | 6 | [114] | |
| EMIM+ | Ac− | 120 | 5 | [114] | |
| EMIM+ | Cl− | 80 | 65 | [114] | |
| EMIM+ | Cl− | 100 | 27 | [114] | |
| EMIM+ | Cl− | 120 | 13 | [114] | |
| EMIM+ | DEP− | 20 | 394 | [115] | |
| EMIM+ | DEP− | 21 | 460 | [116] | |
| EMIM+ | DMP− | 20 | 457 | [115] | |
| EMIM+ | DMP− | 25 | 265 | [117] | |
| EMIM+ | DMP− | 80 | ≈27 | [115] | |
| water | 25 | 0.9 | [118] | ||
| water | 45 | 0.6 | [118] | ||
| DMSO | 25 | 2.0 | [118] | ||
| DMSO | 45 | 1.4 | [118] | ||

3.3. Hydrophobic Reagents in Hydrophilic Solvents—Homogeneous or Heterogeneous Derivatization
3.4. Ionic Liquids as Non-Innocent Solvents—Thermal Stability and Side Reactions

3.5. Toxicity and “Greenness” of Ionic Liquids
3.6. Recyclability of Ionic Liquids

4. Current Developments and Future Perspectives
4.1. Elucidation of the Dissolution Mechanism
| Experimental Technique | Model Compound | Ref. |
|---|---|---|
| computational simulation | glucose, cellodextrins (DP = 2–12) | [174] |
| computational simulation | glucose | [175,176] |
| computational simulation | cellobiose | [177] |
| computational simulation | cellodextrins (DP = 5–20) | [178] |
| computational simulation | (1,4)-dimethoxy-β-D-glucopyranose | [179] |
| computational simulation | cellulose microfibrils (36 glucan chains, DP = 16) | [180,181] |
| computational simulation | cellodextrin (DP = 20) | [182] |
| computational simulation | cellulose microfibrils (36 glucan chains, DP = 20) | [183] |
| computational simulation | cellulose Iβ crystal | [184] |
| computational simulation | cellobiose | [185] |
| computational simulation | (1,4)-dimethoxy-β-D-glucopyranose | [186] |
| IR spectroscopy(computational simulation) | pentaerythritol | [187] |
| neutron diffraction(computational simulation)(NMR spectroscopy) | glucose | [188] |
| NMR spectroscopy | glucose, cellobiose | [169,189,190] |
| NMR spectroscopy | cellobiose | [170] |
| NMR spectroscopy(solvatochromic parameters) | ethanol | [191] |
| solvatochromic parameters | cellulose | [192] |
| solvatochromic parameters | cellulose | [193] |
| solvatochromic parameters | cellulose | [194] |
| X-ray diffraction | cellulose | [195] |
| Ionic liquid a | Solvatochromic parameters b | Dissolves cellulose | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cation | Anion | ETN | α | β | π* | |||||
| AMIM+ | Fo− | n.a. | 0.48 | 0.99 | 1.08 | yes | [198] | |||
| AMIM+ | MPo− | n.a. | 0.51 | 0.99 | 1.06 | yes | [123] | |||
| BMIM+ | Ac− | 0.611 | 0.43 | 1.05 | 1.04 | yes | [199] | |||
| BMIM+ | Ac− | 0.892 | 0.57 | 0.99 | 0.97 | yes | [44] | |||
| BMIM+ | Ac− | n.a. | 0.55 | 1.09 | 0.99 | yes | [113] | |||
| BMIM+ | Ac− | n.a. | 0.43 | 1.20 | n.a. | yes | [193] | |||
| BMIM+ | Ac− | n.a. | 0.36 | 0.85 | n.a. | yes | [200] | |||
| BMIM+ | Ac− | n.a. | n.a. | 1.16 | n.a. | yes | [192] | |||
| BMIM+ | Cl− | 0.901 | 0.51 | 0.84 | 1.08 | yes | [44] | |||
| BMIM+ | Cl− | n.a. | 0.47 | 0.87 | 1.10 | yes | [113] | |||
| BMIM+ | Cl− | n.a. | 0.49 | 0.83 | 1.03 | yes | [193] | |||
| BMIM+ | Fo− | n.a. | 0.56 | 1.01 | 1.03 | yes | [113] | |||
| BMIM+ | MPo− | n.a. | 0.52 | 1.02 | 1.01 | yes | [123] | |||
| EDMIM+ | MPo− | n.a. | 0.33 | 1.01 | 1.11 | yes | [123] | |||
| EMIM+ | DMP− | n.a. | 0.51 | 1.0 | 1.06 | yes | [117] | |||
| EMIM+ | MPo− | n.a. | 0.52 | 1.0 | 1.06 | yes | [123] | |||
| EMPIP+ | MPo− | n.a. | 0.29 | 1.08 | 1.08 | yes | [123] | |||
| HEMIM+ | MPo− | n.a. | 0.63 | 0.91 | 1.06 | yes | [123] | |||
| MOEMIM+ | Ac− | 0.912 | 0.59 | 1.06 | 1.01 | yes | [44] | |||
| MOEMIM+ | MPo− | n.a. | 0.51 | 0.98 | 1.07 | yes | [123] | |||
| TEMA+ | MPo− | n.a. | 0.29 | 1.04 | 1.14 | yes | [123] | |||
| BMIM+ | CH3SO4− | n.a. | 0.54 | 0.67 | 1.05 | no | [193] | |||
| BMIM+ | N(CN)2− | n.a. | 0.44 | 0.64 | n.a. | no | [193] | |||
| BMIM+ | BF4− | 0.670 | 0.63 | 0.38 | 1.05 | no | [199] | |||
| BMIM+ | TfO− | 0.630 | 0.62 | 0.46 | 1.0 | no | [199] | |||
| Molecular solvents | ||||||||||
| methanol | 0.762 | 0.98 | 0.66 | 0.60 | no | [201] | ||||
| DMSO | 0.444 | 0.0 | 0.76 | 1.00 | no | [201] | ||||
| Molecular solvents (cont.) | ||||||||||
| pyridine | 0.302 | 0.0 | 0.64 | 0.87 | no | [201] | ||||
| chloroform | 0.259 | 0.20 | 0.10 | 0.58 | no | [201] | ||||
| toluene | 0.099 | 0.0 | 0.11 | 0.54 | no | [201] | ||||
| hexane | 0.009 | 0.0 | 0.0 | −0.40 | no | [201] | ||||
4.2. Ionic Liquid/Co-Solvent Mixtures as Tailored Reaction Media
4.3. Task Specific Cellulose Solvents for Dissolution, Shaping, and Chemical Modification of Cellulose
4.4. Combination of Derivatization and Shaping of Cellulose in Ionic Liquids
5. Conclusions
Acknowledgments
References and Notes
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of ionic liquids in carbohydrate chemistry: A window of opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T. Interaction of ionic liquids with polysaccharides. 5. Solvents and reaction media for the modification of cellulose. BioResources 2008, 3, 576–601. [Google Scholar]
- Pinkert, A.; Marsh, K.N.; Pang, S.S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Wendler, F.; Kosan, B.; Krieg, M.; Meister, F. Possibilities for the physical modification of cellulose shapes using ionic liquids. Macromol. Symp. 2009, 280, 112–122. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, Y.; Zhang, J.; He, J. Structure and properties of novel regenerated cellulose films prepared from cornhusk cellulose in room temperature ionic liquids. J. Appl. Polym. Sci. 2010, 116, 547–554. [Google Scholar] [CrossRef]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from cellulose-ionic liquid solutions: Preparation, properties and comparison with cellulose-NaOH and cellulose-NMMO routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Gericke, M.; Trygg, J.; Fardim, P. Functional Cellulose beads-prepartion, characterization, and applications. Chem. Rev. 2012. to be submitted for publication. [Google Scholar]
- Stark, A. Ionic liquids in the biorefinery: A critical assessment of their potential. Energ. Environ. Sci. 2011, 4, 19–32. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef]
- Sun, N.; Rodriguez, H.; Rahman, M.; Rogers, R.D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem. Commun. 2011, 47, 1405–1421. [Google Scholar]
- Ionic Liquids in Synthesis, 2nd; Wasserscheid, P.; Welton, T. (Eds.) Wiley-VCH: Weinheim, Germany, 2008.
- Yue, C.; Fang, D.; Liu, L.; Yi, T.-F. Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions. J. Mol. Liq. 2011, 163, 99–121. [Google Scholar] [CrossRef]
- Chaturvedi, D. Recent developments on task specific ionic liquids. Curr. Org. Chem. 2011, 15, 1236–1248. [Google Scholar]
- Buchanan, C.M.; Buchanan, N.L.; Donelson, M.E.; Gorbunova, M.G.; Kuo, T.; Wang, B. Regioselectively substituted cellulose esters produced in a halogenated ionic liquid process and products produced therefrom. WO 2010019245 A1, 2010. [Google Scholar]
- Hembre, R.T.; Buchanan, N.L.; Buchanan, C.M.; Lambert, J.L.; Donelson, M.E.; Gorbunova, M.G.; Kuo, T.; Wang, B. Regioselectively substituted cellulose esters produced in a carboxylated ionic liquid process and products produced therefrom. WO2010019244A1, 2010. [Google Scholar]
- Buchanan, C.M.; Buchanan, N.L.; Hembre, R.T.; Lambert, J.L. Cellulose esters and their production in carboxylated ionic liquids. WO2008100566A1, 2008. [Google Scholar]
- Buchanan, C.; Buchanan, N.L. Reformation of ionic liquids in cellulose esterification. WO2008100569A1, 2008. [Google Scholar]
- Buchanan, C.M.; Buchanan, N.L.; Guzman-Morales, E. Cellulose solutions comprising tetraalkylammonium alkylphosphate and products produced therefrom. WO2010120268A1, 2010. [Google Scholar]
- Stegmann, V.; Massonne, K.; D’Andola, G.; Mormann, W.; Wezstein, M.; Leng, W. Process for acylation of cellulose. DE102006028165A1, 2007. [Google Scholar]
- Massonne, K.; Stegmann, V.; D’Andola, G.; Mormann, W.; Wezstein, M.; Leng, W. Method for acylating polysaccharides or oligosaccharides with a specific average degree of polymerization. WO2008000666A1, 2008. [Google Scholar]
- Liebert, T.; Heinze, T.J. Exploitation of reactivity and selectivity in cellulose functionalization using unconventional media for the design of products showing new superstructures. Biomacromolecules 2001, 2, 1124–1132. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T. Tailored cellulose esters: Synthesis and structure determination. Biomacromolecules 2005, 6, 333–340. [Google Scholar] [CrossRef]
- Verfahren zur Herstellung einer neuen Zelluloselösung und neue Zelluloselösung. CH153446, 1932.
- Graenacher, C. Cellulose Solution. US Patent 1,943,176,1934.
- Husemann, V.E.; Siefert, E. N-Äthyl-pyridinium-chlorid als Lösungsmittel und Reaktionsmedium für Cellulose. Makromol. Chem. 1969, 128, 288–291. [Google Scholar] [CrossRef]
- Linko, Y.-Y.; Viskari, R.; Pohjola, L.; Linko, P. Preparation and performance of cellulose bead-entrapped whole cell glucose isomerase. J. Solid-Phase Biochem. 1977, 2, 203–212. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Electrochemical Aspects of Ionic Liquids, 2nd; Ohno, H. (Ed.) John Wiley & Sons, Inc.: : Hoboken, NJ, USA, 2011.
- Ionic Liquids in Chemical Analysis; Koel, M. (Ed.) CRC Press: Boca Raton, FL, USA, 2009.
- Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T.; Heinze, T.; Edgar, K.J. (Eds.) American Chemical Society: Washington, DC, USA, 2010.
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed.Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry 2. Functionalization of Cellulose; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Heinze, T.; Liebert, T.; Koschella, A. Esterification of Polysaccharides; Springer Verlag: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Barthel, S.; Heinze, T. Acylation and carbanilation of cellulose in ionic liquids. Green Chem. 2006, 8, 301–306. [Google Scholar] [CrossRef]
- Schlufter, K.; Schmauder, H.P.; Dorn, S.; Heinze, T. Efficient homogeneous chemical modification of bacterial cellulose in the ionic liquid 1-N-butyl-3-methylimidazolium chloride. Macromol. Rapid Commun. 2006, 27, 1670–1676. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Owereh, O.S. Homogeneous phase synthesis of cellulose carbamate silica hybrid materials using 1-N-butyl-3-methylimidazolium chloride ionic liquid medium. Carbohydr. Polym. 2009, 78, 635–638. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.; Ren, Q.; Guo, M. Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, J.; Meng, T.; Zhang, J.; He, J.S.; Li, H.Q.; Zhang, Y. Acetone-soluble cellulose acetates prepared by one-step homogeneous acetylation of cornhusk cellulose in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Carbohydr. Polym. 2007, 69, 665–672. [Google Scholar] [CrossRef]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic liquids as reaction medium in cellulose functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef]
- Fidale, L.C.; Possidonio, S.; El Seoud, O.A. Application of 1-Allyl-3-(1-butyl)imidazolium Chloride in the Synthesis of Cellulose Esters: Properties of the Ionic Liquid, and Comparison with Other Solvents. Macromol. Biosci. 2009, 9, 813–821. [Google Scholar] [CrossRef]
- Possidonio, S.; Fidale, L.C.; El Seoud, O.A. Microwave-assisted derivatization of cellulose in an ionic liquid: An efficient, expedient synthesis of simple and mixed carboxylic esters. J. Polym. Sci. Part A 2010, 48, 134–143. [Google Scholar] [CrossRef]
- El Seoud, O.A.; da Silva, V.C.; Possidonio, S.; Casarano, R.; Arêas, E.P.G.; Gimenes, P. Microwave-assisted derivatization of cllulose, 2—The surprising effect of the structure of ionic liquids on the dissolution and acylation of the biopolymer. Macromol. Chem. Physic 2011, 212, 2541–2550. [Google Scholar] [CrossRef]
- Huang, K.; Wang, B.; Cao, Y.; Li, H.; Wang, J.; Lin, W.; Mu, C.; Liao, D. Homogeneous preparation of cellulose acetate propionate (CAP) and cellulose Acetate butyrate (CAB) from sugarcane bagasse cellulose in ionic liquid. J. Agric. Food Chem. 2011, 59, 5376–5381. [Google Scholar]
- Zhang, J.; Wu, J.; Cao, Y.; Sang, S.; Zhang, J.; He, J. Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 2009, 16, 299–308. [Google Scholar] [CrossRef]
- Köhler, S.; Heinze, T. Efficient synthesis of cellulose furoates in 1-N-butyl-3-methylimidazolium chloride. Cellulose 2007, 14, 489–495. [Google Scholar] [CrossRef]
- Huang, K.; Xia, J.; Li, M.; Lian, J.; Yang, X.; Lin, G. Homogeneous synthesis of cellulose stearates with different degrees of substitution in ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2011, 83, 1631–1635. [Google Scholar] [CrossRef]
- Xu, D.; Li, B.; Tate, C.; Edgar, K.J. Studies on regioselective acylation of cellulose with bulky acid chlorides. Cellulose 2011, 18, 405–419. [Google Scholar] [CrossRef]
- Li, W.Y.; Jin, A.X.; Liu, C.F.; Sun, R.C.; Zhang, A.P.; Kennedy, J.F. Homogeneous modification of cellulose with succinic anhydride in ionic liquid using 4-dimethylaminopyridine as a catalyst. Carbohydr. Polym. 2009, 78, 389–395. [Google Scholar] [CrossRef]
- Liu, C.F.; Zhang, A.P.; Li, W.Y.; Yue, F.X.; Sun, R.C. Homogeneous modification of cellulose in ionic liquid with succinic anhydride using N-bromosuccinimide as a catalyst. J. Agric. Food Chem. 2009, 57, 1814–1820. [Google Scholar] [CrossRef]
- Liu, C.F.; Zhang, A.P.; Li, W.Y.; Yue, F.X.; Sun, R.C. Succinoylation of cellulose catalyzed with iodine in ionic liquid. Ind. Crop. Prod. 2010, 31, 363–369. [Google Scholar] [CrossRef]
- Liu, C.F.; Sun, R.C.; Zhang, A.P.; Ren, J.L. Preparation of sugarcane bagasse cellulosic phthalate using an ionic liquid as reaction medium. Carbohyd. Polym. 2007, 68, 17–25. [Google Scholar] [CrossRef]
- Ma, S.; Xue, X.-L.; Yu, S.J.; Wang, Z.H. High-intensity ultrasound irradiated modification of sugarcane bagasse cellulose in an ionic liquid. Ind. Crop. Prod. 2012, 35, 135–139. [Google Scholar] [CrossRef]
- Zarth, C.; Koschella, A.; Pfeifer, A.; Dorn, S.; Heinze, T. Synthesis and characterization of novel amino cellulose esters. Cellulose 2011, 18, 1315–1325. [Google Scholar] [CrossRef]
- Brackhagen, M.; Heinze, T.; Dorn, S.; Koschella, A. Method for manufacturing cellulose derivatives containing amino groups in ionic liquids. EP 2072530 A1, 2009. [Google Scholar]
- Dorn, S.; Pfeifer, A.; Schlufter, K.; Heinze, T. Synthesis of water-soluble cellulose esters applying carboxylic acid imidazolides. Poly. Bull. 2010, 64, 845–854. [Google Scholar] [CrossRef]
- Sui, X.; Yuan, J.; Zhou, M.; Zhang, J.; Yang, H.; Yuan, W.; Wei, Y.; Pan, C. Synthesis of cellulose-graft-poly(N,N-dimethylamino-2-ethyl methacrylate) copolymers via homogeneous ATRP and their aggregates in aqueous media. Biomacromolecules 2008, 9, 2615–2620. [Google Scholar] [CrossRef]
- Meng, T.; Gao, X.; Zhang, J.; Yuan, J.; Zhang, Y.; He, J. Graft copolymers prepared by atom transfer radical polymerization (ATRP) from cellulose. Polymer 2009, 50, 447–454. [Google Scholar] [CrossRef]
- Xin, T.-T.; Yuan, T.; Xiao, S.; He, J. Synthesis of cellulose-graft-poly(methyl methacrylate) via homogeneous ATRP. BioResources 2011, 6, 2941–2953. [Google Scholar]
- Lin, C.-X.; Zhan, H.-Y.; Liu, M.-H.; Fu, S.-Y.; Zhang, J.-J. Preparation of cellulose graft poly(methyl methacrylate) copolymers by atom transfer radical polymerization in an ionic liquid. Carbohydr. Polym. 2009, 78, 432–438. [Google Scholar] [CrossRef]
- Xu, X.; Duan, W.; Huang, M.; Li, G. Synthesis of cellulose dehydroabietate in ionic liquid [bmim]Br. Carbohyd. Res. 2011, 346, 2024–2027. [Google Scholar] [CrossRef]
- Granström, M.; Kavakka, J.; King, A.; Majoinen, J.; Mäkelä, V.; Helaja, J.; Hietala, S.; Virtanen, T.; Maunu, S.-L.; Argyropoulos, D.; et al. Tosylation and acylation of cellulose in 1-allyl-3-methylimidazolium chloride. Cellulose 2008, 15, 481–488. [Google Scholar] [CrossRef]
- Köhler, S.; Liebert, T.; Heinze, T.; Vollmer, A.; Mischnick, P.; Möllmann, E.; Becker, W. Interactions of ionic liquids with polysaccharides 9. Hydroxyalkylation of cellulose without additional inorganic bases. Cellulose 2010, 17, 437–448. [Google Scholar] [CrossRef]
- Möllmann, E.; Heinze, T.; Liebert, T.; Köhler, S. Homogeneous synthesis of cellulose ethers in ionic liquids. US Patent Application 20090221813 A1, 2009. [Google Scholar]
- Granström, M.; Olszewska, A.; Mäkelä, V.; Heikkinen, S.; Kilpeläinen, I. A new protection group strategy for cellulose in an ionic liquid: Simultaneous protection of two sites to yield 2,6-di-O-substituted mono-p-methoxytrityl cellulose. Tetrahedron Lett. 2009, 50, 1744–1747. [Google Scholar]
- Erdmenger, T.; Haensch, C.; Hoogenboom, R.; Schubert, U.S. Homogeneous tritylation of cellulose in 1-butyl-3-methylimidazolium chloride. Macromol. Biosci. 2007, 7, 440–445. [Google Scholar] [CrossRef]
- Myllymaeki, V.; Aksela, R. Etherification of cellulose in ionic liquid solutions. WO2005054298A1, 2005. [Google Scholar]
- Köhler, S.; Liebert, T.; Heinze, T. Interactions of ionic liquids with polysaccharides. VI. Pure cellulose nanoparticles from trimethylsilyl cellulose synthesized in ionic liquids. J. Polym. Sci. Pol. Chem 2008, 46, 4070–4080. [Google Scholar] [CrossRef]
- Mormann, W.; Wezstein, M. Trimethylsilylation of cellulose in ionic liquids. Macromol. Biosci. 2009, 9, 369–375. [Google Scholar] [CrossRef]
- Gericke, M.; Liebert, T.; Heinze, T. Interaction of ionic liquids with polysaccharides, 8—Synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol. Biosci. 2009, 9, 343–353. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T.; Gericke, M. Production water-soluble, low-substituted cellulose sulfates. DE102007035322 B4, 2009. [Google Scholar]
- Wang, Z.-M.; Xiao, K.-J.; Li, L.; Wu, J.-Y. Molecular weight-dependent anticoagulation activity of sulfated cellulose derivatives. Cellulose 2010, 17, 953–961. [Google Scholar] [CrossRef]
- Gericke, M.; Schaller, J.; Liebert, T.; Fardim, P.; Meister, F.; Heinze, T. Studies on the tosylation of cellulose in mixtures of ionic liquids and a co-solvent. Carbohydr. Polym. 2012. [Google Scholar] [CrossRef]
- Granström, M.; Mormann, W.; Frank, P. Method for chlorinating polysaccharides or oligosaccharides. WO2011086082 A1, 2011. [Google Scholar]
- Yan, C.; Zhang, J.; Lv, Y.; Yu, J.; Wu, J.; Zhang, J.; He, J. Thermoplastic cellulose-graft-poly(l-lactide) copolymers homogeneously synthesized in an ionic liquid with 4-dimethylaminopyridine catalyst. Biomacromolecules 2009, 10, 2013–2018. [Google Scholar] [CrossRef]
- Lin, C.-X.; Zhan, H.-Y.; Liu, M.-H.; Fu, S.-Y.; Huang, L.-H. Rapid homogeneous preparation of cellulose graft copolymer in BMIMCL under microwave irradiation. J. Appl. Polym. Sci. 2010, 118, 399–404. [Google Scholar]
- Hao, Y.; Peng, J.; Li, J.; Zhai, M.; Wei, G. An ionic liquid as reaction media for radiation-induced grafting of thermosensitive poly (N-isopropylacrylamide) onto microcrystalline cellulose. Carbohydr.Polym. 2009, 77, 779–784. [Google Scholar] [CrossRef]
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddart, B. O-Acetylation of cellulose and monosaccharides using a zinc based ionic liquid. Green Chem. 2005, 7, 705–707. [Google Scholar] [CrossRef]
- Eastman Chemical Company. Available online: http://www.eastman.com/Literature_Center/E/E325.pdf (accessed on 1 June 2011).
- Celanese Corporation Home Page. Available online: http://www.celanese.com/index/productsmarkets_index/products_markets_acetate.html (accessed on 1 June 2011).
- Hussain, M.A.; Liebert, T.; Heinze, T. Acylation of cellulose with N,N′-carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol.Rapid Commun. 2004, 25, 916–920. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Torenius, H.M.; Tye, H. Microwave-promoted organic synthesis using ionic liquids: A mini review. Comb. Chem. High T. Scr. 2004, 7, 511–528. [Google Scholar]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U. S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodriguez, H.; Rogers, R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Hoffmann, J.; Nuchter, M.; Ondruschka, B.; Wasserscheid, P. Ionic liquids and their heating behaviour during microwave irradiation - a state of the art report and challenge to assessment. Green Chem. 2003, 5, 296–299. [Google Scholar] [CrossRef]
- Gericke, M.; Liebert, T.; Heinze, T. Polyelectrolyte synthesis and in situ complex formation in ionic liquids. J. Am. Chem. Soc. 2009, 131, 13220–13221. [Google Scholar] [CrossRef]
- Dow Chemical Company Home Page. Available online: http://www.dow.com/assets/attachments/industry/building_construction/Cellosize_brochure.pdf (accessed on 12 August 2011).
- Gómez, J.A.C.; Erler, U.W.; Klemm, D.O. 4-Methoxy substituted trityl groups in 6-O protection of cellulose: Homogeneous synthesis, characterization, detritylation. Macromol.Chem. Phys. 1996, 197, 953–964. [Google Scholar] [CrossRef]
- Kondo, T.; Gray, D.G. The preparation of O-methyl- and O-ethyl-celluloses having controlled distribution of substituents. Carbohyd. Res. 1991, 220, 173–183. [Google Scholar] [CrossRef]
- Petzold-Welcke, K.; Kötteritzsch, M.; Heinze, T. 2,3-O-Methyl cellulose: Studies on synthesis and structure characterization. Cellulose 2010, 17, 449–457. [Google Scholar] [CrossRef]
- Saake, B.; Patt, R.; Puls, J.; Philipp, B. Molecular weight distribution of cellulose. Papier 1991, 45, 727–735. [Google Scholar]
- Aparicio, S.; Atilhan, M.; Karadas, F. Thermophysical properties of pure ionic liquids: Review of present situation. Ind. Eng. Chem. Res. 2010, 49, 9580–9595. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Ab Rani, M.A.; Brant, A.; Crowhurst, L.; Dolan, A.; Lui, M.; Hassan, N.H.; Hallett, J.P.; Hunt, P.A.; Niedermeyer, H.; Perez-Arlandis, J.M.; et al. Understanding the polarity of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 16831–16840. [Google Scholar]
- Gallo, V.; Mastrorilli, P.; Nobile, C.F.; Romanazzi, G.; Suranna, G.P. How does the presence of impurities change the performance of catalytic systems in ionic liquids? A case study: the Michael addition of acetylacetone to methyl vinyl ketone. J. Chem. Soc. Dalton 2002, 23, 4339–4342. [Google Scholar]
- Lee, S.; Ha, S.; Lee, S.; Koo, Y.-M. Adverse effect of chloride impurities on lipase-catalyzed transesterifications in ionic liquids. Biotechnol. Lett. 2006, 28, 1335–1339. [Google Scholar] [CrossRef]
- Gordon, C.M.; Muldoon, M.J.; Wagner, M.; Hilgers, C.; Davis, J.H.; Wasserscheid, P. Synthesis and Purification. In Ionic Liquids in Synthesis, 2nd; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 7–55. [Google Scholar]
- Stark, A.; Behrend, P.; Braun, O.; Müller, A.; Ranke, J.; Ondruschka, B.; Jastorff, B. Purity specification methods for ionic liquids. Green Chem. 2008, 10, 1152–1161. [Google Scholar] [CrossRef]
- Clare, B.; Sirwardana, A.; MacFarlane, D. Synthesis, Purification and Characterization of Ionic Liquids. In Ionic Liquids: Topics in Current Chemistry; Kirchner, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 290, pp. 1–40. [Google Scholar]
- Sigma Aldrich Home Page. Available online: http://www.sigmaaldrich.com (accessed on 1 June 2012).
- IoLiTec GmbH Home Page. Available online: http://www.iolitec.de/en/ (accessed on 1 June 2012).
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solution thermodynamics of imidazolium-based ionic liquids and Water. J. Phys. Chem. B 2001, 105, 10942–10949. [Google Scholar]
- Mazza, M.; Catana, D.-A.; Vaca-Garcia, C.; Cecutti, C. Influence of water on the dissolution of cellulose in selected ionic liquids. Cellulose 2009, 16, 207–215. [Google Scholar] [CrossRef]
- Le, K.; Sescousse, R.; Budtova, T. Influence of water on cellulose-EMIMAc solution properties: A viscometric study. Cellulose 2012, 19, 45–54. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, J.; Zhang, J.; Niu, Y.; Liu, C.-Y.; He, J.; Zhang, J. Rheological properties of cellulose/ionic liquid/dimethylsulfoxide (DMSO) solutions. Polymer 2012, 53, 2524–2531. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Lee, Y.-Y.; Peng, W.-H.; Wu, K.C.W. Cellulosic conversion in ionic liquids (ILs): Effects of H2O/cellulose molar ratios, temperatures, times, and different ILs on the production of monosaccharides and 5-hydroxymethylfurfural (HMF). Catal. Today 2011, 174, 65–69. [Google Scholar]
- Zhang, Z.; Wang, W.; Liu, X.; Wang, Q.; Li, W.; Xie, H.; Zhao, Z.K. Kinetic study of acid-catalyzed cellulose hydrolysis in 1-butyl-3-methylimidazolium chloride. Bioresour. Technol. 2012, 112, 151–155. [Google Scholar] [CrossRef]
- Ha, S. H.; Mai, N.L.; An, G.; Koo, Y.-M. Microwave-assisted pretreatment of cellulose in ionic liquid for accelerated enzymatic hydrolysis. Biores. Technol. 2011, 102, 1214–1219. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, C.L.; Baker, G.A.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009, 139, 47–54. [Google Scholar]
- Gericke, M.; Schlufter, K.; Liebert, T.; Heinze, T.; Budtova, T. Rheological properties of cellulose/ionic liquid solutions: from dilute to concentrated states. Biomacromolecules 2009, 10, 1188–1194. [Google Scholar] [CrossRef]
- Sescousse, R.; Le, K.A.; Ries, M.E.; Budtova, T. Viscosity of cellulose-imidazolium-based ionic liquid solutions. J. Phys. Chem. B 2010, 114, 7222–7228. [Google Scholar]
- Chen, Y.; Zhang, Y.; Ke, F.; Zhou, J.; Wang, H.; Liang, D. Solubility of neutral and charged polymers in ionic liquids studied by laser light scattering. Polymer 2011, 52, 481–488. [Google Scholar] [CrossRef]
- Ohno, H.; Fukaya, Y. Task specific ionic liquids for cellulose technology. Chem. Lett. 2009, 38, 2–7. [Google Scholar] [CrossRef]
- Fendt, S.; Padmanabhan, S.; Blanch, H.W.; Prausnitz, J.M. Viscosities of acetate or chloride-based ionic liquids and some of their mixtures with water or other common solvents. J. Chem. Eng. Data 2010, 56, 31–34. [Google Scholar]
- Kuhlmann, E.; Himmler, S.; Giebelhaus, H.; Wasserscheid, P. Imidazolium dialkylphosphates-a class of versatile, halogen-free and hydrolytically stable ionic liquids. Green Chem. 2007, 9, 233–242. [Google Scholar] [CrossRef]
- Vitz, J.; Yevlampieva, N.P.; Rjumtsev, E.; Schubert, U.S. Cellulose molecular properties in 1-alkyl-3-methylimidazolium-based ionic liquid mixtures with pyridine. Carbohydr. Polym. 2010, 82, 1046–1053. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Carmen Grande, M.d.; Juliá, J.A.; García, M.; Marschoff, C.M. On the density and viscosity of (water+dimethylsulphoxide) binary mixtures. J. Chem. Thermodyn. 2007, 39, 1049–1056. [Google Scholar] [CrossRef]
- Gericke, M. Cellulose Sulfates Prepared in Ionic Liquids-From Challenging Synthesis to Promising Biomedical Applications; Der Andere Verlag: Uelvesbüll, Germany , 2010. [Google Scholar]
- Lovell, C.S.; Walker, A.; Damion, R.A.; Radhi, A.; Tanner, S.F.; Budtova, T.; Ries, M.E. Influence of cellulose on ion diffusivity in 1-ethyl-3-methyl-imidazolium acetate cellulose solutions. Biomacromolecules 2010, 11, 2927–2935. [Google Scholar] [CrossRef]
- Inczédy, J.; Lengyel, T.; Ure, A.M. Compendium of Analytical Nomenclature: Definitive Rules, 3rd ed; Blackwell Science: Oxford, UK, 1998. [Google Scholar]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Kim Seung, S.; Ohno, H. Design of Polar Ionic Liquids to Solubilize Cellulose without Heating. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T., Heinze, T., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1033, pp. 55–66. [Google Scholar]
- Rahn, K.; Diamantoglou, M.; Klemm, D.; Berghmans, H.; Heinze, T. Homogeneous synthesis of cellulose p-toluenesulfonates in N,N-dimethylacetamide/LiCl solvent system. Angew. Makromol. Chem. 1996, 238, 143–163. [Google Scholar] [CrossRef]
- Gericke, M.; Liebert, T.; El Seoud, O.A.; Heinze, T. Tailored media for homogeneous cellulose chemistry: Ionic liquid/co-solvent mixtures. Macromol. Mater. Eng. 2011, 296, 483–493. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mohan, R.S.; Scott, J.L. Reactivity of ionic liquids. Tetrahedron 2007, 63, 2363–2389. [Google Scholar] [CrossRef]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.-C.; Chu, Y.-H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef]
- Alder, R.W.; Allen, P.R.; Williams, S.J. Stable carbenes as strong bases. J. Chem. Soc. Chem. Commun. 1995, 12, 1267–1268. [Google Scholar]
- Amyes, T.L.; Diver, S.T.; Richard, J.P.; Rivas, F.M.; Toth, K. Formation and stability of N-heterocyclic carbenes in water: The carbon acid pKa of imidazolium cations in aqueous solution. J. Am. Chem. Soc. 2004, 126, 4366–4374. [Google Scholar] [CrossRef]
- Canal, J.P.; Ramnial, T.; Dickie, D.A.; Clyburne, J.A.C. From the reactivity of N-heterocyclic carbenes to new chemistry in ionic liquids. Chem. Commun. 2006, 17, 1809–1818. [Google Scholar]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef]
- Rodriguez, H.; Gurau, G.; Holbrey, J.D.; Rogers, R.D. Reaction of elemental chalcogens with imidazolium acetates to yield imidazole-2-chalcogenones: Direct evidence for ionic liquids as proto-carbenes. Chem. Commun. 2011, 47, 3222–3224. [Google Scholar]
- Kelemen, Z.; Holloczki, O.; Nagy, J.; Nyulaszi, L. An organocatalytic ionic liquid. Org. Biomol. Chem. 2011, 9, 5362–5364. [Google Scholar] [CrossRef]
- Ebner, G.; Schiehser, S.; Potthast, A.; Rosenau, T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008, 49, 7322–7324. [Google Scholar] [CrossRef]
- Handy, S.T.; Okello, M. The 2-Position of imidazolium ionic liquids: Substitution and exchange. J. Org. Chem. 2005, 70, 1915–1918. [Google Scholar] [CrossRef]
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar] [CrossRef]
- Dorn, S.; Wendler, F.; Meister, F.; Heinze, T. Interactions of ionic liquids with polysaccharides-7: Thermal stability of cellulose in ionic liquids and N-methylmorpholine-N-oxide. Macromol. Mater. Eng. 2008, 293, 907–913. [Google Scholar] [CrossRef]
- Wendler, F.; Todi, L.N.; Meister, F. Thermostability of imidazolium ionic liquids as direct solvents for cellulose. Thermochim. Acta 2012, 528, 76–84. [Google Scholar] [CrossRef]
- Wendler, F.; Graneß, G.; Heinze, T. Characterization of autocatalytic reactions in modified cellulose/NMMO solutions by thermal analysis and UV/VIS spectroscopy. Cellulose 2005, 12, 411–422. [Google Scholar] [CrossRef]
- Wendler, F.; Konkin, A.; Heinze, T. Studies on the stabilization of modified Lyocell solutions. Macromol. Symp. 2008, 262, 72–84. [Google Scholar] [CrossRef]
- Kroon, M.C.; Buijs, W.; Peters, C.J.; Witkamp, G.-J. Quantum chemical aided prediction of the thermal decomposition mechanisms and temperatures of ionic liquids. Thermochim. Acta 2007, 465, 40–47. [Google Scholar] [CrossRef]
- Chambreau, S.D.; Boatz, J.A.; Vaghjiani, G.L.; Koh, C.; Kostko, O.; Golan, A.; Leone, S.R. Thermal decomposition mechanism of 1-ethyl-3-methylimidazolium bromide ionic liquid. J. Phys. Chem. A 2011. [Google Scholar] [CrossRef]
- Liebner, F.; Patel, I.; Ebner, G.; Becker, E.; Horix, M.; Potthast, A.; Rosenau, T. Thermal aging of 1-alkyl-3-methylimidazolium ionic liquids and its effect on dissolved cellulose. Holzforschung 2010, 64, 161–166. [Google Scholar] [CrossRef]
- Köhler, S.; Liebert, T.; Schöbitz, M.; Schaller, J.; Meister, F.; Günther, W.; Heinze, T. Interactions of ionic liquids with polysaccharides 1. Unexpected acetylation of cellulose with 1-ethyl-3-methylimidazolium acetate. Macromol. Rapid Commun. 2007, 28, 2311–2317. [Google Scholar] [CrossRef]
- Karatzos, S.; Edye, L.; Wellard, R. The undesirable acetylation of cellulose by the acetate ion of 1-ethyl-3-methylimidazolium acetate. Cellulose 2012, 19, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Thuy Pham, T.P.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Silva Pereira, C. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef]
- Frade, R.F.M.; Afonso, C.A.M. Impact of ionic liquids in environment and humans: An overview. Hum. Exp. Toxicol. 2010, 29, 1038–1054. [Google Scholar] [CrossRef]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure-property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Matzke, M.; Stolte, S.; Thiele, K.; Juffernholz, T.; Arning, J.; Ranke, J.; Welz-Biermann, U.; Jastorff, B. The influence of anion species on the toxicity of 1-alkyl-3-methylimidazolium ionic liquids observed in an (eco)toxicological test battery. Green Chem. 2007, 9, 1198–1207. [Google Scholar] [CrossRef]
- Merck Ionic Liquids Biological Effects Database. Available online: http://www.il-eco.uft.uni-bremen.de/ (accessed on 1 February 2012).
- Ropel, L.; Belvèze, L.S.; Aki, S.N.V.K.; Stadtherr, M.A.; Brennecke, J.F. Octanol-water partition coefficients of imidazolium-based ionic liquids. Green Chem. 2005, 7, 83–90. [Google Scholar] [CrossRef]
- Coleman, D.; Gathergood, N. Biodegradation studies of ionic liquids. Chem. Soc. Rev. 2010, 39, 600–637. [Google Scholar] [CrossRef]
- Stolte, S.; Arning, J.; Thöming, J. Biodegradability of ionic liquids—Test pocedures and structural design. Chem. Ing. Tech. 2011, 83, 1454–1467. [Google Scholar]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R. Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem. 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Righi, S.; Morfino, A.; Galletti, P.; Samori, C.; Tugnoli, A.; Stramigioli, C. Comparative cradle-to-gate life cycle assessments of cellulose dissolution with 1-butyl-3-methylimidazolium chloride and N-methyl-morpholine-N-oxide. Green Chem. 2011, 13, 367–375. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Progr. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Chuman, H. Toward basic understanding of the partition coefficient log P and its application in QSAR. SAR QSAR Environ. Res. 2008, 19, 71–79. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar]
- Ventura, S.P.M.; Sousa, S.G.; Serafim, L.S.; Lima, Á.S.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid based aqueous biphasic systems with controlled pH: The ionic liquid cation effect. J. Chem. Eng. Data 2011, 56, 4253–4260. [Google Scholar] [CrossRef]
- Todi, L.N.; Wendler, F.; Kraemer, A.; Meister, F.; Heinze, T. Extended characterization of various cellulose/ionic liquid systems. In Presented at the 243rd ACS National Meeting & Exposition, San Diego, CA, USA, 25–29 March 2012.
- Zhao, H.; Brown, H.; Holladay, J.; Zhang, Z. Prominent roles of impurities in ionic liquid for catalytic conversion of carbohydrates. Top. Catal. 2012, 55, 33–37. [Google Scholar]
- Villagrán, C.; Deetlefs, M.; Pitner, W.R.; Hardacre, C. Quantification of halide in ionic liquids using ion chromatography. Anal. Chem. 2004, 76, 2118–2123. [Google Scholar] [CrossRef]
- Haerens, K.; Van Deuren, S.; Matthijs, E.; Van der Bruggen, B. Challenges for recycling ionic liquids by using pressure driven membrane processes. Green Chem. 2010, 12, 2182–2188. [Google Scholar] [CrossRef]
- Han, S.; Wong, H.T.; Livingston, A.G. Application of organic solvent Nanofiltration to separation of ionic liquids and products from ionic liquid mediated reactions. Chem. Eng. Res. Des. 2005, 83, 309–316. [Google Scholar] [CrossRef]
- Esperanca, J.M.S.S.; Canongia Lopes, J.N.; Tariq, M.; Santos, L.M.N.B.F.; Magee, J.W.; Rebelo, L.P.N. Volatility of aprotic ionic liquids—A review. J. Chem. Eng. Data 2009, 55, 3–12. [Google Scholar]
- King, A.W.T.; Asikkala, J.; Mutikainen, I.; Järvi, P.; Kilpeläinen, I. Distillable acid-base conjugate ionic liquids for cellulose dissolution and processing. Angew. Chem. 2011, 50, 6301–6305. [Google Scholar] [CrossRef]
- Remsing, R.C.; Hernandez, G.; Swatloski, R.P.; Massefski, W.W.; Rogers, R.D.; Moyna, G. Solvation of carbohydrates in N,N′-dialkylimidazolium ionic liquids: A multinuclear NMR spectroscopy study. J. Phys. Chem. B 2008, 112, 11071–11078. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.; He, J.; Xiang, J. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1941–1947. [Google Scholar]
- Remsing, R.C.; Petrik, I.D.; Liu, Z.; Moyna, G. Comment on “NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids” by J. Zhang, H. Zhang, J. Wu, J. Zhang, J. He and J. Xiang, Phys. Chem. Chem. Phys., 2010, 12, 1941. Phys. Chem. Chem. Phys. 2010, 12, 14827–14828. [Google Scholar]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.; He, J.; Xiang, J. Reply to “Comment on ‘NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids’” by R.C. Remsing, I.D. Petrik, Z.Liu and G. Moyna, Phys. Chem. Chem. Phys., 2010, 12, doi:10.1039/c004203j. Phys. Chem. Chem. Phys. 2010, 12, 14829–14830. [Google Scholar]
- Vitz, J.; Erdmenger, T.; Schubert, U.S. Imidazolium based ionic liquids as solvents for cellulose chemistry. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T., Heinze, T., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1033, pp. 55–66. [Google Scholar]
- Derecskei, B.; Derecskei-Kovacs, A. Molecular dynamic studies of the compatibility of some cellulose derivatives with selected ionic liquids. Mol. Simulat. 2006, 32, 109–115. [Google Scholar] [CrossRef]
- Youngs, T.G.A.; Holbrey, J.D.; Deetlefs, M.; Nieuwenhuyzen, M.; Costa Gomes, M.F.; Hardacre, C. A molecular dynamics study of glucose solvation in the ionic liquid 1,3-dimethylimidazolium chloride. ChemPhysChem 2006, 7, 2279–2281. [Google Scholar] [CrossRef]
- Youngs, T.G.A.; Hardacre, C.; Holbrey, J.D. Glucose solvation by the ionic liquid 1,3-dimethylimidazolium chloride: A simulation study. J. Phys. Chem. B 2007, 111, 13765–13774. [Google Scholar] [CrossRef]
- Novoselov, N.; Sashina, E.; Petrenko, V.; Zaborsky, M. Study of dissolution of cellulose in ionic liquids by computer modeling. Fibre Chem. 2007, 39, 153–158. [Google Scholar] [CrossRef]
- Liu, H.; Sale, K.L.; Holmes, B.M.; Simmons, B.A.; Singh, S. Understanding the interactions of cellulose with ionic liquids: A molecular dynamics study. J. Phys. Chem. B 2010, 114, 4293–4301. [Google Scholar]
- Janesko, B.G. Modeling interactions between lignocellulose and ionic liquids using DFT-D. Phys. Chem. Chem. Phys. 2011, 13, 11393–11401. [Google Scholar] [CrossRef]
- Cho, H.M.; Gross, A.S.; Chu, J.-W. Dissecting force interactions in cellulose deconstruction reveals the required solvent versatility for overcoming biomass recalcitrance. J. Am. Chem. Soc. 2011, 133, 14033–14041. [Google Scholar] [CrossRef]
- Gross, A.S.; Bell, A.T.; Chu, J.-W. Thermodynamics of cellulose solvation in water and the ionic liquid 1-butyl-3-methylimidazolim chloride. J. Phys. Chem. B 2011, 115, 13433–13440. [Google Scholar] [CrossRef]
- Liu, H.; Sale, K.L.; Simmons, B. A.; Singh, S. Molecular dynamics study of polysaccharides in binary solvent mixtures of an ionic liquid and water. J. Phys. Chem. B 2011, 115, 10251–10258. [Google Scholar]
- Mostofian, B.; Smith, J.; Cheng, X. The solvation structures of cellulose microfibrils in ionic liquids. Interdiscip.Sci. Comput. Life Sci. 2011, 3, 308–320. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Mechanistic understanding of interactions between cellulose and ionic liquids: A molecular simulation study. Polymer 2011, 52, 5904–5911. [Google Scholar] [CrossRef]
- Payal, R.S.; Bharath, R.; Periyasamy, G.; Balasubramanian, S. Density functional theory investigations on the structure and dissolution mechanisms for cellobiose and xylan in an ionic liquid: Gas phase and cluster calculations. J. Phys. Chem. B 2011, 116, 833–840. [Google Scholar]
- Ding, Z.-D.; Chi, Z.; Gu, W.-X.; Gu, S.-M.; Liu, J.-H.; Wang, H.-J. Theoretical and experimental investigation on dissolution and regeneration of cellulose in ionic liquid. Carbohydr. Polym. 2012, 89, 7–16. [Google Scholar] [CrossRef]
- Papanyan, Z.; Roth, C.; Paschek, D.; Ludwig, R. Understanding the dissolution of polyols by ionic liquids using the example of a well-defined model compound. ChemPhysChem 2011, 12, 2400–2404. [Google Scholar] [CrossRef]
- Youngs, T.G.A.; Holbrey, J.D.; Mullan, C.L.; Norman, S.E.; Lagunas, M.C.; D’Agostino, C.; Mantle, M.D.; Gladden, L.F.; Bowron, D.T.; Hardacre, C. Neutron diffraction, NMR and molecular dynamics study of glucose dissolved in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Chem. Sci. 2011, 2, 1594–1605. [Google Scholar] [CrossRef]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-N-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl-NMR relaxation study on model systems. Chem. Commun. 2006, 12, 1271–1273. [Google Scholar]
- Liu, Z.; Remsing Richard, C.; Moore Preston, B.; Moyna, G. Molecular dynamics study of the mechanism of cellulose dissolution in the ionic liquid l-N-butyl-3-methylimidazolium chloride. In Ionic Liquids IV Not Just Solvents Anymore; Brennecke1, J.F., Rogers, R.D., Seddon, K.R., Eds.; American Chemical Society: Washington, DC, USA, 2007; Volume 975, pp. 335–350. [Google Scholar]
- Sellin, M.; Ondruschka, B.; Stark, A. Hydrogen Bond Acceptor Properties of Ionic Liquids and Their Effect on Cellulose Solubility. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T., Heinze, T., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1033, pp. 121–135. [Google Scholar]
- Xu, A.R.; Wang, J.J.; Wang, H.Y. Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem. 2010, 12, 268–275. [Google Scholar] [CrossRef]
- Brandt, A.; Hallett, J.P.; Leak, D. J.; Murphy, R.J.; Welton, T. The effect of the ionic liquid anion in the pretreatment of pine wood chips. Green Chem. 2010, 12, 672–679. [Google Scholar] [CrossRef]
- Rinaldi, R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef]
- Samayam, I.P.; Hanson, B.L.; Langan, P.; Schall, C.A. Ionic-liquid induced changes in cellulose structure associated with enhanced biomass hydrolysis. Biomacromolecules 2011, 12, 3091–3098. [Google Scholar] [CrossRef]
- Meiland, M.; Liebert, T.; Baumgaertel, A.; Schubert, U.; Heinze, T. Alkyl β-D-cellulosides: Non-reducing cellulose mimics. Cellulose 2011, 18, 1585–1598. [Google Scholar] [CrossRef]
- Sixta, H. Novel Aspects Applied in Cellulose Chemistry and Technology. In Presented at the Polysaccharides as Source of Advanced and Sustainable Products, 2nd International Polysaccharide Conference EPNOE 2011, Wageningen, The Netherlands, 29 August–2 September 2011.
- Fukaya, Y.; Sugimoto, A.; Ohno, H. Superior solubility of polysaccharides in low viscosity, polar, and halogen-free 1,3-dialkylimidazolium formates. Biomacromolecules 2006, 7, 3295–3297. [Google Scholar] [CrossRef]
- Wu, Y.S.; Sasaki, T.; Kazushi, K.; Seo, T.; Sakurai, K. Interactions between spiropyrans and room-temperature ionic liquids: Photochromism and solvatochromism. J. Phys. Chem. B 2008, 112, 7530–7536. [Google Scholar]
- Lungwitz, R.; Friedrich, M.; Linert, W.; Spange, S. New aspects on the hydrogen bond donor (HBD) strength of 1-butyl-3-methylimidazolium room temperature ionic liquids. New J. Chem. 2008, 32, 1493–1499. [Google Scholar] [CrossRef]
- Marcus, Y. The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 1993, 22, 409–416. [Google Scholar]
- Lv, Y.; Wu. J, Zhang, J.; Niu, Y., Liu; Zhang, J. Rheological properties of cellulose/ionic liquid/dimethylsulfoxide (DMSO) solutions. Polymer 2012. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M. The effect of subcritical carbon dioxide on the dissolution of cellulose in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Cellulose 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Matsumoto, H.; Tatsumi, K. Low-melting, low-viscous, hydrophobic ionic liquids: Aliphatic quaternary ammonium salts with perfluoroalkyltrifluoroborates. Chem. Eur. J. 2005, 11, 752–766. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, S.; Li, Z.; Zhang, Q.; Deng, Y. Dialkoxy functionalized quaternary ammonium ionic liquids as potential electrolytes and cellulose solvents. New J. Chem. 2011, 35, 1596–1606. [Google Scholar] [CrossRef]
- Köhler, S.; Liebert, T.; Heinze, T. Ammonium-based cellulose solvents suitable for homogeneous etherification. Macromol. Biosci. 2009, 9, 836–841. [Google Scholar] [CrossRef]
- Hummel, M.; Laus, G.; Schwärzler, A.; Bentivoglio, G.; Rubatscher, E.; Kopacka, H.; Wurst, K.; Kahlenberg, V.; Gelbrich, T.; Griesser Ulrich, J.; et al. Non-halide ionic liquids for solvation, extraction, and processing of cellulosic materials. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T., Heinze, T., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1033, pp. 229–259. [Google Scholar]
- D’Andola, G.; Szarvas, L.; Massonne, K.; Stegmann, V. Ionic liquids for solubilizing polymers. WO2008043837A1, 2008. [Google Scholar]
- Hummel, M.; Froschauer, C.; Laus, G.; Roder, T.; Kopacka, H.; Hauru, L.K.J.; Weber, H.K.; Sixta, H.; Schottenberger, H. Dimethyl phosphorothioate and phosphoroselenoate ionic liquids as solvent media for cellulosic materials. Green Chem. 2011, 13, 2507–2517. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Koech, P.K.; Ang, M.T.C.; Liang, C.; Rainbolt, J.E.; Yonker, C.R.; Jessop, P.G. Reversible zwitterionic liquids, the reaction of alkanol guanidines, alkanol amidines, and diamines with CO2. Green Chem. 2010, 12, 713–721. [Google Scholar] [CrossRef]
- Jessop, P.G.; Phan, L.; Carrier, A.; Robinson, S.; Durr, C.J.; Harjani, J.R. A solvent having switchable hydrophilicity. Green Chem. 2010, 12, 809–814. [Google Scholar] [CrossRef]
- Anugwom, I.; Mäki-Arvela, P.; Virtanen, P.; Willför, S.; Sjöholm, R.; Mikkola, J.P. Selective extraction of hemicelluloses from spruce using switchable ionic liquids. Carbohyd. Polym. 2012, 87, 2005–2011. [Google Scholar] [CrossRef]
- Kosan, B.; Dorn, S.; Meister, F.; Heinze, T. Preparation and subsequent shaping of cellulose acetates using ionic liquids. Macromol. Mater. Eng. 2010, 295, 676–681. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gericke, M.; Fardim, P.; Heinze, T. Ionic Liquids — Promising but Challenging Solvents for Homogeneous Derivatization of Cellulose. Molecules 2012, 17, 7458-7502. https://doi.org/10.3390/molecules17067458
Gericke M, Fardim P, Heinze T. Ionic Liquids — Promising but Challenging Solvents for Homogeneous Derivatization of Cellulose. Molecules. 2012; 17(6):7458-7502. https://doi.org/10.3390/molecules17067458
Chicago/Turabian StyleGericke, Martin, Pedro Fardim, and Thomas Heinze. 2012. "Ionic Liquids — Promising but Challenging Solvents for Homogeneous Derivatization of Cellulose" Molecules 17, no. 6: 7458-7502. https://doi.org/10.3390/molecules17067458
APA StyleGericke, M., Fardim, P., & Heinze, T. (2012). Ionic Liquids — Promising but Challenging Solvents for Homogeneous Derivatization of Cellulose. Molecules, 17(6), 7458-7502. https://doi.org/10.3390/molecules17067458