Ultrasound-Promoted One-Pot, Four-Component Synthesis of Pyridin-2(1H)-One Derivatives
Abstract
:1. Introduction
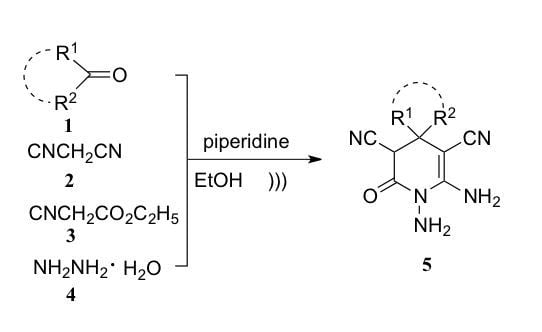
2. Results and Discussion

| Entry | Solvent | Temperature (°C) | Catalyst | Time (min) | Isolated Yield (%) |
|---|---|---|---|---|---|
| 1 | EtOH | rt | No catalyst | 600 | trace |
| 2 | EtOH | rt | Piperidine (10%) | 30 | 93 |
| 3 | EtOH | rt | NaOH (10%) | 240 | 80 |
| 4 | EtOH | rt | KOH (10%) | 180 | 78 |
| 5 | EtOH | rt | Na2CO3 (10%) | 360 | 62 |
| 6 | EtOH | rt | EtONa (10%) | 240 | 83 |
| 7 | EtOH | 40 | Piperidine (10%) | 30 | 89 |
| 8 | EtOH | 50 | Piperidine (10%) | 30 | 85 |
| 9 | MeOH | rt | Piperidine (10%) | 40 | 85 |
| 10 | CH3CN | rt | Piperidine (10%) | 120 | 65 |
| 11 | THF | rt | Piperidine (10%) | 90 | 79 |
| 12 | Water | rt | Piperidine (10%) | 120 | trace |
| 13 | EtOH | rt | Piperidine (5%) | 60 | 86 |
| 14 | EtOH | rt | Piperidine (15%) | 60 | 90 |
| 15 | EtOH | rt | Piperidine (20%) | 30 | 91 |
| 16 | EtOH | rt | Piperidine (25%) | 30 | 89 |

| Entry | Ketone | Product | With US | Without US | ||
|---|---|---|---|---|---|---|
| Time (min) | Yield (%) | Time (min) | Yield (%) | |||
| 1 |  |  | 30 | 94 | 120 | 72 |
| 2 |  |  | 30 | 92 | 120 | 69 |
| 3 |  |  | 30 | 93 | 180 | 70 |
| 4 |  |  | 30 | 92 | 180 | 60 |
| 5 |  |  | 35 | 92 | 180 | 65 |
| 6 |  |  | 40 | 91 | 180 | 67 |
| 7 |  |  | 40 | 90 | 180 | 62 |
| 8 |  |  | 40 | 91 | 240 | 59 |
| 9 |  |  | 40 | 89 | 300 | 60 |
| 10 |  |  | 35 | 88 | 300 | 60 |
| 11 |  |  | 35 | 89 | 180 | 64 |


3. Experimental
3.1. General Information
3.2. General Procedure for the Synthesis of 1,6-Diamino-2-oxo-1,2,3,4-Tetrahydro-Pyridine-3,5-Dicarbonitrile Derivatives 5
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nair, V.; Rajesh, C.; Vinod, A.U.; Bindu, S.; Sreekanth, A.R.; Mathen, J.S.; Balagopal, L. Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes. Acc. Chem. Res. 2003, 36, 899–907. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Dömling, A. recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005; pp. 33–75. [Google Scholar]
- Jiang, B.; Tu, S.J.; Kaur, P.; Wever, W.; Li, G. Four-component domino reaction leading to multifunctionalized quinazolines. J. Am. Chem. Soc. 2009, 131, 11660–11661. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Y.; Xie, Y.J.; Yan, C.G. Facile synthesis of dispirooxindole-fused heterocycles via domino 1,4-dipolar addition and Diels-Alder reaction of in situ generated Huisgen 1,4-dipoles. Org. Lett. 2012, 14, 5172–5175. [Google Scholar] [CrossRef]
- Li, M.; Cao, H.; Wang, Y.; Lv, X.L.; Wen, L.R. One-pot multicomponent cascade reaction of N,S-ketene acetal: Solvent-free synthesis of imidazo[1,2-a]thiochromeno[3,2-e]pyridines. Org. Lett. 2012, 14, 3470–3473. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Lin, W.; Dou, G.L.; Huang, Z.B.; Shi, D.Q. Highly efficient synthesis of polysubstituted pyrroles via four-component domino reaction. Org. Lett. 2013, 15, 2542–2545. [Google Scholar] [CrossRef]
- Cao, C.P.; Lin, W.; Hu, M.H.; Huang, Z.B.; Shi, D.Q. Highly efficient construction of pentacyclic benzo[b]indeno[1,2,3-de][1,8]naphthyridine derivatives via four-component domino reaction. Chem. Commun. 2013, 49, 6983–6985. [Google Scholar]
- Franklin, E.C. Heterocyclic nitrogen compounds. I. Petnacyclic compounds. Chem. Rev. 1935, 16, 305–361. [Google Scholar] [CrossRef]
- Bergstrom, F.W. Heterocyclic nitrogen compounds. Part IIA. Hexacyclic compounds: Pyridine, quinoline, and isoquinoline. Chem. Rev. 1944, 35, 77–277. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W. Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc. Chem. Res. 2002, 35, 728–737. [Google Scholar] [CrossRef]
- Dobbin, P.S.; Hider, R.C.; Hall, A.D.; Taylor, P.D.; Sarpong, P.; Porter, J.B. Synthesis, physicochemical properties, and biological evaluation of N-substituted 2-alkyl-3-hydroxy-4(1H)-pyridinones: Orally active iron chelators with clinical potential. J. Med. Chem. 1993, 36, 2448–2458. [Google Scholar] [CrossRef]
- Rai, B.L.; Dekhordi, L.S.; Khodr, H.; Jin, Y.; Liu, Z.; Hider, R.C. Synthesis, physicochemical properties, and evaluation of N-substituted-2-alkyl-3-hydroxy-4(1H)-pyridinones. J. Med. Chem. 1998, 41, 3347–3359. [Google Scholar] [CrossRef]
- Mosti, L.; Schenone, P.; Menozzi, G. Reaction of 2-dimethylaminomethylene-1,3-diones with dinucleophilies. V. Synthesis of 5-acyl-1,2-dihydro-2-oxo-3-pyridinecarbonitriles and 1,2,5,6,7,8-hexahydro-2,5-dioxo-3-quinolinecarboxamides. J. Heterocycl. Chem. 1985, 22, 1503–1509. [Google Scholar] [CrossRef]
- Mosti, L.; Menozzi, G.; Schenone, P.; Dorigo, P.; Gaion, R.M.; Benetollo, F.; Bombieri, G. Synthesis and cardiotonic activity of esters of 2-substituted 5-cyano-1,6-dihydro-6-oxo-3-pyridinecarboxylic acids. Crystal structure of 2-methyl, 2-t-butyl and 2-phenyl esters. Eur. J. Med. Chem. 1989, 24, 517–529. [Google Scholar] [CrossRef]
- Nelson, W.O.; Karpishin, T.B.; Rettig, S.J.; Orvig, C. Physical and structural studies of N-substituted-3-hydroxy-2-methyl-4(1H)-pyridinones. Can. J. Chem. 1988, 66, 123–131. [Google Scholar] [CrossRef]
- Zhang, Z.; Rettig, S.J.; Orvig, C. Lipophilic coordination compounds: Aluminum, gallium, and indium complexs of 1-aryl-3-hydroxy-2-methyl-4-pyridinones. Inorg. Chem. 1991, 30, 509–515. [Google Scholar] [CrossRef]
- Stoncius, S.; Orentas, E.; Butkus, E.; Öhrström, L.; Wendt, O.F.; Wämmark, K. An approach to helical tubular self-aggregation using C2-symmetric self-complementary hydrogen-bonding cavity molecules. J. Am. Chem. Soc. 2006, 128, 8272–8285. [Google Scholar]
- Cocco, M.T.; Congiu, C.; Onnis, V. Synthesis and antitumour activity of 4-hydroxy-2-pyridone derivatives. Eur. J. Med. Chem. 2000, 35, 545–552. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V. New bis(pyridyl)methane derivatives from 4-hydroxy-2-pyridones: Synthesis and antitumoral activity. Eur. J. Med. Chem. 2003, 38, 37–47. [Google Scholar] [CrossRef]
- Cella, R.; Stefani, H.A. Ultrasound in heterocycles chemistry. Tetrahedron 2009, 65, 2619–2641. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Forcing and controlling chemical reactions with ultrasound. Angew. Chem. Int. Ed. 2007, 46, 5476–5478. [Google Scholar] [CrossRef]
- Li, J.T.; Sun, M.X.; Yin, Y. Ultrasound promoted efficient method for the cleavage of 3-aryl-2,3-epoxyl-1-phenyl-1-propanone with indole. Ultrason. Sonochem. 2010, 17, 359–362. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, H.; Hu, Y.; Liu, H.; Zhao, X.; Ji, H.L.; Shi, D.Q. A novel and environment-friendly method for preparing dihydropyrano[2,3-c]pyrazoles in water under ultrasound irradiation. Ultrason. Sonochem. 2011, 18, 708–712. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Liu, H.; Shi, D.Q. Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] derivatives. ACS Comb. Sci. 2012, 14, 38–43. [Google Scholar] [CrossRef]
- Hu, Y.; Zou, Y.; Wu, H.; Shi, D.Q. A facile and efficient ultrasound-assisted synthesis of novel dispiroheterocycles through 1,3-dipolar cycloaddition reactions. Ultrason. Sonochem. 2012, 19, 264–269. [Google Scholar] [CrossRef]
- Shi, D.Q.; Zou, Y.; Hu, Y.; Wu, H. Imporved synthesis of dihydrothiophenes derivatives under ultrasound irradiation. J. Hetreocycl. Chem. 2011, 48, 896–900. [Google Scholar] [CrossRef]
- Liu, H.; Zou, Y.; Hu, Y.; Shi, D.Q. An efficient one-pot synthesis of dispiropyrrolidine derivatives through 1,3-dipolar cycloaddition reactions under ultrasound irradiation. J. Heterocycl. Chem. 2011, 48, 877–881. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zou, Y.; Zhao, X.; Shi, D.Q. A novel and convenient synthesis of 4-hydroxy-6-methyl-3-(1-(phenylimino)ethyl)-2H-pyran-2-one derivatives under ultrasound irradiation. Ultrason. Sonochem. 2011, 18, 1048–1051. [Google Scholar]
- Lortente, A.; Galan, C.; Fonseca, I.; Sanz-Aparicio, J. 1-Aminocyclohexene-2,4-dicarbonitrile derivatives. Synthesis and structural study. Can. J. Chem. 1995, 73, 1546–1555. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5a–k are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, J.; Li, Q.; Zhang, J.; Lin, W.; Wang, J.; Wang, Y.; Huang, Z.; Shi, D. Ultrasound-Promoted One-Pot, Four-Component Synthesis of Pyridin-2(1H)-One Derivatives. Molecules 2013, 18, 14519-14528. https://doi.org/10.3390/molecules181214519
Yang J, Li Q, Zhang J, Lin W, Wang J, Wang Y, Huang Z, Shi D. Ultrasound-Promoted One-Pot, Four-Component Synthesis of Pyridin-2(1H)-One Derivatives. Molecules. 2013; 18(12):14519-14528. https://doi.org/10.3390/molecules181214519
Chicago/Turabian StyleYang, Jinming, Qiang Li, Juanjuan Zhang, Wei Lin, Juxian Wang, Yucheng Wang, Zhibin Huang, and Daqing Shi. 2013. "Ultrasound-Promoted One-Pot, Four-Component Synthesis of Pyridin-2(1H)-One Derivatives" Molecules 18, no. 12: 14519-14528. https://doi.org/10.3390/molecules181214519
APA StyleYang, J., Li, Q., Zhang, J., Lin, W., Wang, J., Wang, Y., Huang, Z., & Shi, D. (2013). Ultrasound-Promoted One-Pot, Four-Component Synthesis of Pyridin-2(1H)-One Derivatives. Molecules, 18(12), 14519-14528. https://doi.org/10.3390/molecules181214519