Two New Koumine-Type Indole Alkaloids from Gelsemium elegans Benth.
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
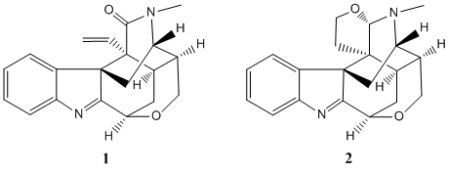
3.4. Characterization of 21-Oxokoumine (1)
3.5. Characterization of Furanokoumine (2)
4. Conclusions
Acknowledgments
References
- Yamada, Y.; Kitajima, M.; Kogure, N.; Takayama, H. Four novel gelsedine-type oxindole alkaloids from Gelsemium elegans. Tetrahedron 2008, 64, 7690–7694. [Google Scholar] [CrossRef]
- Dutt, V.; Thakur, S.; Dhar, V.J.; Sharma, A. The genus Gelsemium: An update. Pharmacogn. Rev. 2010, 4, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Wang, L.; Zhang, Q.W.; Wang, G.C.; Wang, Y.; Huang, X.J.; Zhang, X.Q.; Jiang, R.W.; Yao, X.S.; Che, C.T.; et al. Six new monoterpenoid indole alkaloids from the aerial part of Gelsemium elegans. Tetrahedron 2011, 67, 4807–4813. [Google Scholar] [CrossRef]
- Liang, S.; He, C.Y.; Szabó, L.F.; Feng, Y.; Lin, X.; Wang, Y. Gelsochalotine, a novel indole ring-degraded monoterpenoid indole alkaloid from Gelsemium elegans. Tetrahedron Lett. 2013, 54, 887–890. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, Y.H.; Zhang, Q.; Yan, X.H.; Di, Y.T.; He, H.P.; Hao, X.J. Three novel β-carboline alkaloids from Gelsemium elegans. Fitoterapia 2012, 83, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Nakamura, T.; Kogure, N.; Ogawa, M.; Mitsuno, Y.; Ono, K.; Yano, S.; Aimi, N.; Takayama, H. Isolation of gelsedine-type indole alkaloids from Gelsemium elegans and evaluation of the cytotoxic activity of gelsemium alkaloids for A431 epidermoid carcinoma cells. J. Nat. Prod. 2006, 69, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Cordell, G.A.; Ni, C.Z.; Clardy, J. 19-(R)- and 19-(S)-Hydroxydihydrokoumine from Gelsemium elegans. Phytochemistry 1990, 29, 965–968. [Google Scholar] [CrossRef]
- Ponglux, D.; Wongseripipatana, S.; Takayama, H.; Ogata, K.; Aimi, N.; Sakai, S.I. A new class of indole alkaloid ‘elegansamine’ constructed from a monoterpenoid indole alkaloid and an iridoid. Tetrahedron Lett. 1988, 29, 5395–5396. [Google Scholar] [CrossRef]
- Ponglux, D.; Wongseripipatana, S.; Subhadhirasakul, S.; Takayama, H.; Yokota, M.; Ogata, K.; Phisalaphong, C.; Aimi, N.; Sakai, S.I. Studies on the indole alkaloids of gelsemium elegans (Thailand): Structure elucidation and proposal of biogenetic route. Tetrahedron 1988, 44, 5075–5094. [Google Scholar] [CrossRef]
- Zhang, Z.; Di, Y.T.; Wang, Y.H.; Mu, S.Z.; Fang, X.; Zhang, Y.; Tan, C.J.; Zhang, Q.; Yan, X.H.; Guo, J.; et al. Gelegamines A–E: Five new oxindole alkaloids from Gelsemium elegans. Tetrahedron 2009, 65, 4551–4556. [Google Scholar] [CrossRef]
- Yamada, Y.; Kitajima, M.; Kogure, N.; Wongseripipatana, S.; Takayama, H. Seven new monoterpenoid indole alkaloids from Gelsemium elegans. Chem. Asian J. 2011, 6, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; He, X.F.; Wu, Y.; Yue, J.M. Monoterpenoid indole alkaloids bearing an N4-Iridoid from Gelsemium elegans. Chem. Asian J. 2008, 3, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |



| 21-oxokoumine (1) | furanokoumine (2) | |||
|---|---|---|---|---|
| Position | δH | δC | δH | δC |
| 2 | 183.1 | 185.5 | ||
| 3 | 5.04 (1H, m) | 70.7 | 5.02 (1H, m) | 71.3 |
| 5 | 3.64 (1H, m) | 58.3 | 2.88 (1H, m) | 59.7 |
| 6 | 2.75 (1H, dd, J = 13.8, 3.0 Hz) | 36.9 | 2.37 (1H, overlapped) | 27.5 |
| 2.09 (1H, br d, J = 13.8 Hz) | 2.28 (1H, dd, J = 14.5, 3.2 Hz) | |||
| 7 | 57.4 | 57.2 | ||
| 8 | 142.7 | 142.1 | ||
| 9 | 7.18 (1H, d, J = 7.6 Hz) | 122.8 | 7.50 (1H, d, J = 7.6 Hz) | 123.0 |
| 10 | 7.25 (1H, td, J = 7.6, 0.8 Hz) | 126.9 | 7.27 (1H, td, J = 7.6, 0.8 Hz) | 126.0 |
| 11 | 7.35 (1H, td, J = 7.6, 0.8 Hz) | 128.5 | 7.38 (1H, td, J = 7.6, 0.8 Hz) | 128.7 |
| 12 | 7.61 (1H, d, J = 7.6 Hz) | 121.2 | 7.62 (1H, d, J = 7.6 Hz) | 121.5 |
| 13 | 154.5 | 155.2 | ||
| 14 | 2.61 (1H, dt, J = 14.7, 3.9 Hz) | 25.4 | 2.72 (1H, dt, J = 14.7, 3.8 Hz) | 25.8 |
| 2.08 (1H, br d J = 14.7 Hz) | 1.70 (1H, dt, J = 14.7, 2.2 Hz) | |||
| 15 | 2.71 (1H, m) | 37.9 | 2.38 (1H, overlapped) | 25.3 |
| 16 | 2.52 (1H, br d, J = 12.0 Hz) | 31.4 | 2.78 (1H, m) | 40.7 |
| 17 | 4.25 (1H, dd, J = 12.2, 4.3 Hz) | 60.7 | 4.2 (1H, dd, J = 12.1, 4.7 Hz) | 61.4 |
| 3.69 (1H, d, J = 12.2 Hz) | 3.62 (1H, d, J = 12.1 Hz) | |||
| 18 | 5.13 (1H, dd, J = 8.2, 4.2 Hz) | 119.8 | 3.86 (1H, q-like, J = 8.0 Hz) | 64.1 |
| 4.83 (1H, overlapped) | 3.60 (1H, dt, J = 4.0, 9.5 Hz) | |||
| 19 | 4.84 (1H, overlapped) | 132.3 | 1.23 (1H, ddd, J = 12.5, 8.4 Hz) | 26.6 |
| 0.91 (1H, ddd, J = 12.5, 9.5, 8.0 Hz) | ||||
| 20 | 53.9 | 51.2 | ||
| 21 | 171.8 | 4.60 (1H, s) | 98.3 | |
| N-Me | 3.20 (3H, s) | 32.4 | 2.74 (3H, s) | 41.0 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, M.; Hou, X.; Gao, H.; Guo, J.; Xiao, K. Two New Koumine-Type Indole Alkaloids from Gelsemium elegans Benth. Molecules 2013, 18, 1819-1825. https://doi.org/10.3390/molecules18021819
Sun M, Hou X, Gao H, Guo J, Xiao K. Two New Koumine-Type Indole Alkaloids from Gelsemium elegans Benth. Molecules. 2013; 18(2):1819-1825. https://doi.org/10.3390/molecules18021819
Chicago/Turabian StyleSun, Mingxue, Xiaoli Hou, Huanhuan Gao, Junsheng Guo, and Kai Xiao. 2013. "Two New Koumine-Type Indole Alkaloids from Gelsemium elegans Benth." Molecules 18, no. 2: 1819-1825. https://doi.org/10.3390/molecules18021819
APA StyleSun, M., Hou, X., Gao, H., Guo, J., & Xiao, K. (2013). Two New Koumine-Type Indole Alkaloids from Gelsemium elegans Benth. Molecules, 18(2), 1819-1825. https://doi.org/10.3390/molecules18021819