The Reaction of Ethyl 2-oxo-2H-chromene-3-carboxylate with Hydrazine Hydrate
Abstract
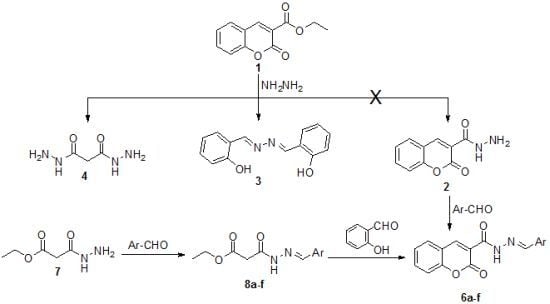
:1. Introduction
2. Results and Discussion




3. Experimental
3.1. General
3.2. The Reaction of Ethyl 2-oxo-2H-Chromene-3-carboxylate (1) with Hydrazine Hydrate
3.3. General Procedure for the Synthesis of Ethyl 3-(2-arylidenehydrazinyl)-3-oxopropanoates 8a–f
3.4. General Procedure for the Synthesis of N'-Arylidene-2-oxo-2H-chromene-3-carbohydrazides 6a–f
3.5. Direct Synthesis of 6f
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from Dr. Hatem A. Abdel-Aziz, Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia.
References
- Fang, G.-M.; Li, Y.-M.; Shen, F.; Huang, Y.-C.; Li, J.-B.; Lin, Y.; Cui, H.-K.; Liu, L. Protein chemical synthesis by ligation of peptide hydrazides. Angew. Chem. Int. Ed. 2011, 50, 7645–7649. [Google Scholar]
- Hassan, A.A.; Shawky, A.M. Chemistry and heterocyclization of carbohydrazides. J. Het. Chem. 2010, 47, 745–763. [Google Scholar] [CrossRef]
- Attanasi, O.A.; Filippone, P.; Perrulli, F.R.; Santeusanio, S. Regioselective role of the hydrazide moiety in the formation of complex pyrrole-pyrazole systems. Tetrahedron 2001, 57, 1387–1394. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Abuo-Rahma, G.A.; Hassan, A.A. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur. J. Med. Chem. 2009, 44, 3480–3487. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E.; Scialino, G. Synthesis and antimycobacterial activity of (3,4-diaryl-3H-thiazol-2-ylidene)-hydrazide derivatives. IL Farmaco 2003, 58, 631–637. [Google Scholar] [CrossRef]
- Jha, K.K.; Samad, A.; Kumar, Y.; Shaharyar, M.; Khosa, R.L.; Jain, J.; Kumar, V.; Singh, P. Design, synthesis and biological evaluation of 1,3,4-oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 4963–4967. [Google Scholar] [CrossRef]
- Yeung, K.-S.; Farkas, M.E.; Kadow, J.F.; Meanwell, N.A. A base-catalyzed, direct synthesis of 2,5-disubstituted 1,2,4-triazoles from nitriles and hydrazides. Tetrahedron Lett. 2005, 46, 3429–3432. [Google Scholar] [CrossRef]
- Farshori, N.N.; Banday, M.R.; Ahmad, A.; Khan, A.U.; Rauf, A. Synthesis, characterization, and in vitro antimicrobial activities of 5-alkenyl/hydroxyalkenyl-2-phenylamine-1,3,4-oxadiazoles and thiadiazoles. Bioorg. Med. Chem. Lett. 2010, 20, 1933–1938. [Google Scholar] [CrossRef]
- Almajan, G.L.; Barbuceanu, S.-F.; Bancescu, G.; Saramet, I.; Saramet, G.; Draghici, C. Synthesis and antimicrobial evaluation of some fused heterocyclic [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives. Eur. J. Med Chem. 2010, 45, 6139–6146. [Google Scholar] [CrossRef]
- Skoumbourdis, A.P.; Huang, R.; Southall, N.; Leister, W.; Guo, V.; Cho, M.-H.; Inglese, J.; Nirenberg, M.; Austin, C.P.; Xia, M.; et al. Identification of a potent new chemotype for the selective inhibition of PDE4. Bioorg. Med. Chem. Lett. 2008, 18, 1297–1303. [Google Scholar]
- Saha, A.; Kumar, R.; Kumar, R.; Devakumar, C. Development and assessment of green synthesis of hydrazides. Indian J. Chem. 2010, 49B, 526–531. [Google Scholar]
- Aboul-Fadl, T.; Abdel-Aziz, H.A.; Kadi, A.; Bari, A.; Ahmad, P.; Al-Samani, T.; Ng, S.W. Microwave-assisted one-step synthesis of fenamic acid hydrazides from the corresponding acids. Molecules 2011, 16, 3544–3551. [Google Scholar] [CrossRef]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Bizzarri, B.; Granese, A.; Carradori, S.; Yáñez, M.; Orallo, F.; Ortuso, F.; et al. Synthesis, molecular modeling, and selective inhibitory activity against human monoamine oxidases of 3-carboxamido-7-substituted coumarins. J. Med. Chem. 2009, 52, 1935–1942. [Google Scholar]
- Traven, V.F. New synthetic routes to furocoumarins and their analogs: A review. Molecules 2004, 9, 50–66. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Fun, H.-K.; Attia, M.I.; Chia, T.S.; Elsaman, T.; Abdel-Aziz, H.A. 2-(2,3-Dimethylanilino)benzohydrazide. Acta Cryst. 2012, E68, o2527–o2528. [Google Scholar]
- Abdel-Aziz, H.A.; Abdel-Wahab, B.F.; Badria, F.A. Stereoselective synthesis and antiviral activity of (1E,2Z,3E)-1-(piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol-2-oyl)hydrazono]-4-(aryl1)but-3-enes. Arch. Pharm. 2010, 343, 152–159. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Mekawey, A.A.I.; Dawood, K.M. Convenient synthesis and antimicrobial evaluation of some novel 2-substituted-3-methylbenzofuran derivatives. Eur. J. Med. Chem. 2009, 44, 3637–3644. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Gamal-Eldeen, A.M.; Hamdy, N.A.; Fakhr, I.M.I. Immunomodulatory and anti-cancer activity of some novel 2-substituted-6-bromo-3-methylthiazolo[3,2-a]benzimidazole derivatives. Arch. Pharm. 2009, 342, 230–237. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of some new 1,3-thiazole, 1,3,4-thiadiazole, 1,2,4-triazole and [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine derivatives including 5-(benzofuran-2-yl)-1-phenyl-pyrazole moiety. Monatsh. Chem. 2009, 140, 601–605. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Convenient synthesis and antimicrobial activity of some new 3-substituted-5-(benzofuran-2-yl)-pyrazole derivatives. Arch. Pharm. 2008, 341, 734–739. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Hamdy, N.A.; Farag, A.M.; Fakhr, I.M.I. Synthesis and reactions of 3-methylthiazolo[3,2-a]benzimidazole-2-carboxylic acid hydrazide: Synthesis of some new pyrazole, 1,3-thiazoline, 1,2,4-triazole and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives pendant to thiazolo[3,2-a]benzimidazole moiety. J. Chin. Chem. Soc. 2007, 54, 1573–1582. [Google Scholar]
- Dawood, K.M.; Farag, A.M.; Abdel-Aziz, H.A. Synthesis of some new benzofuran-based thiophene, 1,3-oxathiole and 1,3,4-oxa(thia)diazole derivatives. Heteroatom Chem. 2007, 18, 294–300. [Google Scholar] [CrossRef]
- Dawood, K.M.; Farag, A.M.; Abdel-Aziz, H.A. Synthesis and antimicrobial evaluation of some 1,2,4-triazole, 1,3,4-oxa(thia)diazole and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives. Heteroatom Chem. 2005, 16, 621–627. [Google Scholar] [CrossRef]
- Bhalla, M.; Shukla, S.; Gujrati, V.R.; Saxena, A.K.; Sanger, K.C.; Shaker, K. Anti-inflammatory benzopyran-2-ones and their active oxygen species (aos) scavenging activity. Boll. Chim. Farm. 1998, 137, 403–411. [Google Scholar]
- Bhat, M.A.; Siddiqui, N.; Khan, S.A. Synthesis of novel 3-(4-acetyl-5-H/methyl-5-substituted phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)-2H-chromen-2-ones as potential anticonvulsant agents. Acta Pol. Pharm. Drug Res. 2008, 65, 235–239. [Google Scholar]
- Bhalla, M.; Hitkari, A.; Gujrati, V.R.; Bhalla, T.N.; Shanker, K. Benzopyran-2-one derivatives: Antiinflammatory, analgesic and antiproteolytic agents. Eur. J. Med. Chem. 1994, 29, 713–717. [Google Scholar] [CrossRef]
- Raslan, M.A.; Khalil, M.A. Heterocyclic synthesis containing bridgehead nitrogen atom: Synthesis of 3-[(2H)-2-oxobenzo[b]pyran-3-yl]-s-triazolo[3,4-b]-1,3,4-thiadiazine and thiazole derivatives. Heteroatom Chem. 2003, 14, 114–120. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Akhtar, M. Synthesis of some new 2,5-disubstituted 1,3,4-oxadiazole derivatives and their biological activity. Indian J. Chem. 2003, 42B, 900–904. [Google Scholar]
- RamaGanesh, C.K.; Bodke, Y.D.; Venkatesh, K.B. Synthesis and biological evaluation of some innovative coumarin derivatives containing thiazolidin-4-one ring. Indian J. Chem. 2010, 49B, 1151–1154. [Google Scholar]
- Singh, V.; Srivastava, V.K.; Palit, G.; Shanker, K. Coumarin congeners as antidepressants. Arzneim-Forsch. Drug Res. 1992, 42, 993–996. [Google Scholar]
- Sivakumar, K.K.; Rajasekaran, A.; Ponnilavarasan, I.; Somasundaram, A.; Sivasakthi, R.; Kamalaveni, S. Synthesis and evaluation of anti-microbial and analgesic activity of some (4Z)-3-methyl-1-[(2-oxo-2H-chromen-4-yl)carbonyl]-1H-pyrazole-4,5-dione-4-[(4-substitutedphenyl)hydrazone]. Der Pharmacia Lett. 2010, 2, 211–219. [Google Scholar]
- Cardoso, S.H.; Barreto, M.B.; Lourenço, M.C.S.; das Graças, M.; Henriques, M.D.O.; Candéa, A.L.P.; Kaiser, C.R.; de Souza, M.V.N. Antitubercular activity of new coumarins. Chem. Biol. Drug Des. 2011, 77, 489–493. [Google Scholar] [CrossRef]
- Patil, S.A.; Unki, S.N.; Kulkarni, A.D.; Naik, V.H.; Badami, P.S. Synthesis, characterization, in vitro antimicrobial and DNA cleavage studies of Co(II), Ni(II) and Cu(II) complexes with ONOO donor coumarin Schiff bases. J. Mol. Struct. 2011, 985, 330–338. [Google Scholar] [CrossRef]
- Sreeja, S.; Mathan, S.; Kumaran, J. Design, synthesis and pharmacological evaluation of new coumarin derivatives. Int. J. Adv. Pharm. Biol. Sci. 2012, 2, 80–91. [Google Scholar]
- Biradar, J.S.; Manjunath, S.Y. Synthesis of novel 2-(5'-substituted-2'-phenylindole-3'-yl)-5-(coumarin-3''-yl)-1,3,4-oxadiazoles and 4-(5'-substituted-2'-phenylindole-3'-yl)-1-(coumarin-3''-amido)azetidin-2-one and their antimicrobial activity. Indian J. Chem. 2004, 43B, 141–143. [Google Scholar]
- Islam, A.M.; Bedair, A.H.; Aly, F.M.; El-Sharief, A.M.Sh.; El-Masry, F.M. Synthesis and reactions of coumarin-3-N-bromo-arylcarboxamides. Indian J. Chem. 1980, 19B, 224–227. [Google Scholar]
- Soliman, F.S.G.; Labouta, I.M.; Stadlbauer, W. Reactions of some coumarins with hydrazine and phenyl hydrazine. Arch. Pharm. Chim. 1985, 13, 49–52. [Google Scholar]
- Li, D.; Tan, M.X.; Jie, L. Synthesis, antioxidant and antibacterial activities of salicylaldehyde azine. Adv. Mat. Res. 2012, 396–398, 2366–2369. [Google Scholar]
- Metwally, S.A.M.; AbdelMoneim, M.I.; Elossely, Y.A.; Awad, R.I.; Abou-Hadeed, K. Synthesis and crystal structure of some 3,5-pyrazolidinediones. Chem. Het. Comp. 2010, 46, 426–437. [Google Scholar]
- Rozin, Yu.A.; Vorob’ova, E.A.; Morzherin, Yu.Yu.; Bakulev, V.A. Synthesis and investigation of ring-chain isomerism of the derivatives of N-amino-5-hydroxy-1,2,3-triazole -4-carboxylic acid. Chem. Het. Comp. 2001, 37, 294–304. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdel-Aziz, H.A.; Elsaman, T.; Attia, M.I.; Alanazi, A.M. The Reaction of Ethyl 2-oxo-2H-chromene-3-carboxylate with Hydrazine Hydrate. Molecules 2013, 18, 2084-2095. https://doi.org/10.3390/molecules18022084
Abdel-Aziz HA, Elsaman T, Attia MI, Alanazi AM. The Reaction of Ethyl 2-oxo-2H-chromene-3-carboxylate with Hydrazine Hydrate. Molecules. 2013; 18(2):2084-2095. https://doi.org/10.3390/molecules18022084
Chicago/Turabian StyleAbdel-Aziz, Hatem A., Tilal Elsaman, Mohamed I. Attia, and Amer M. Alanazi. 2013. "The Reaction of Ethyl 2-oxo-2H-chromene-3-carboxylate with Hydrazine Hydrate" Molecules 18, no. 2: 2084-2095. https://doi.org/10.3390/molecules18022084
APA StyleAbdel-Aziz, H. A., Elsaman, T., Attia, M. I., & Alanazi, A. M. (2013). The Reaction of Ethyl 2-oxo-2H-chromene-3-carboxylate with Hydrazine Hydrate. Molecules, 18(2), 2084-2095. https://doi.org/10.3390/molecules18022084