Simultaneous Determination of Multiple Sesquiterpenes in Curcuma wenyujin Herbal Medicines and Related Products with One Single Reference Standard
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Sample Pre-Treatment
2.2. Validation of the Method
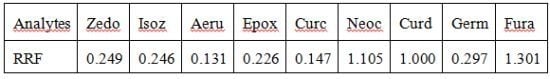
2.3. Relative Response Factors
2.4. Ruggedness and Robustness Tests of RRF
2.5. Quantitative Measurement of Different Samples from C. wenyujin
3. Experimental
3.1. Materials and Reagents
3.2. Instruments and Chromatographic Conditions
3.3. Sample Preparation
3.4. Sesquiterpene Standards Solution Preparation
3.5. Calculation of Relative Response Factors and Quantification of Sesquiterpenes
4. Conclusions
Acknowledgments
References
- Xiao, Y.; Yang, F.Q.; Li, S.P.; Hu, G.; Lee, S.M.Y.; Wang, Y.T. Essential oil of Curcuma wenyujin induces apoptosis in human hepatoma cells. World J. Gastroenterol. 2008, 14, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Zheng, Y.P.; Liu, Z.F.; Xu, L.L.; Li, S.H. Studies on the chemical constituents of sesquiterpenoids from Curcuma wenyujin. J. Instrum. Anal. 2006, 25, 27–30. [Google Scholar]
- Xia, Q.; Huang, Z.G.; Li, S.P.; Zhang, P.; Wang, J.; He, L.N. The experiment study of anti-virus effects of zedoary oil on influenza virus and respiratory syncytial virus. Chin. Pharm. Bull. 2004, 20, 357–358. [Google Scholar]
- Ge, Y.W.; Gao, H.M.; Wang, Z.M. Advances in study of genus Curcuma. China J. Chin. Mater. Med. 2007, 32, 2461–2467. [Google Scholar]
- Adio, A.M. Germacrenes A-E and related compounds: thermal, photochemical and acid induced transannular cyclizations. Tetrahedron 2009, 65, 1533–1552. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Shirota, O.; Sekita, S.; Nakane, T. Transannular cyclization of (4S,5S)-Germacrone-4,5-epoxide into guaiane and secoguaiane-type sesquiterpenes. Nat. Prod. Commun. 2012, 7, 441–446. [Google Scholar] [PubMed]
- Baldovini, N.; Tomi, F.; Casanova, J. Identification and quantitative determination of furanodiene, a heat-sensitive compound, in essential oil by 13C-NMR. Phytochem. Anal. 2001, 12, 58–63. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall-Weyerstahl, H.; Christiansen, C.; Oguntimein, B.O.; Adeoye, A.O. Volatile constituents of Eugenia uniflora leaf oil. Planta Med. 1988, 54, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Li, S.P.; Cheng, Y.; Lao, S.C.; Wang, Y.T. Optimization of GC-MS conditions based on resolution and stability of analytes for simultaneous determination of nine sesquiterpenoids in three species of Curcuma rhizomes. J. Pharm. Biomed. Anal. 2007, 43, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Wang, Y.T.; Li, S.P. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2006, 1134, 226–231. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeial Convention. The United States Pharmacopoeia; United Book Press: Rockville, MD, USA, 2011; Volume 34, pp. 1072–1073. [Google Scholar]
- European Pharmacopeia Convention. European Pharmacopeia; European Directorate for the Quality of Medicines & HealthCare Press: Strasbourg, France, 2006; pp. 436–437. [Google Scholar]
- The Pharmacopoeia Committee of China. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 69, 257–258. [Google Scholar]
- Wang, H.F.; Provan, G.J.; Helliwell, K. HPLC determination of catechins in tea leaves and tea extracts using relative response factors. Food Chem. 2003, 81, 307–312. [Google Scholar] [CrossRef]
- Feng, W.H.; Wang, Z.M.; Zhang, Q.W.; Liu, L.M.; Wang, J.Y.; Yang, F. Quantitative method for simultaneous assay of four coumarins with one marker in fraxini cortex. Chin. J. Chin. Mater. Med. 2011, 36, 1782–1789. [Google Scholar]
- Gao, X.Y.; Jiang, Y.; Lu, J.Q.; Tu, P.F. One single standard substance for the determination of multiple anthraquinone derivatives in rhubarb using high-performance liquid chromatography-diode array detection. J. Chromatogr. A 2009, 1216, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Wang, Z.M.; Kang, Y.H.; Zhang, Q.W.; Gao, Q.P.; Ma, N. A quantitative method using one marker for simultaneous assay of ginsenosides in Panax ginseng and P. notoginseng. Acta Pharm. Sin. 2008, 43, 1211–1216. [Google Scholar]
- Hou, J.J.; Wu, W.Y.; Da, J.; Yao, S.; Long, H.L.; Yang, Z.; Cai, L.Y.; Yang, M.; Liu, X.; Jiang, B.H.; et al. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J. Chromatogr. A 2011, 1218, 5618–5627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Wang, Z.M.; Zhang, Q.W.; Li, F. A quantitative method for simultaneous assay of four flavones with one marker in Radix scutellaria. China J. Chin. Mater. Med. 2009, 34, 3229–3234. [Google Scholar]
Sample Availability: Samples of the compounds zedoarondiol, isozedoarondiol, aerugidiol, (4S,5S)-(+)-germacrone-4,5-epoxide, curcumenone, neocurdione are available from the authors. |


| Analyte | Calibration equation | Linear range (µg) | r2 | LOD (ng) | LOQ (ng) |
|---|---|---|---|---|---|
| Zedo | y = 9.56 × 106x + 1.59 × 103 | 0.0236–1.18 | 0.9999 | 0.94 | 2.40 |
| Isoz | y = 9.77 × 106x + 4.36 × 102 | 0.0081–0.81 | 1.0000 | 0.81 | 2.71 |
| Aeru | y = 1.88 × 107x – 2.15 × 103 | 0.0040–0.40 | 0.9999 | 0.40 | 1.32 |
| Epox | y = 1.05 × 107x + 9.74 × 103 | 0.0066–6.58 | 1.0000 | 2.60 | 6.60 |
| Curc | y = 1.61 × 107x + 9.52 × 103 | 0.0445–4.45 | 1.0000 | 0.74 | 2.23 |
| Neoc | y = 2.31 × 106x – 2.43 × 103 | 0.0586–2.93 | 0.9999 | 1.20 | 2.90 |
| Curd | y = 2.41 × 106x + 3.06 × 103 | 0.296–14.79 | 1.0000 | 2.40 | 5.91 |
| Germ | y = 8.04 × 106x + 2.34 × 103 | 0.059–2.98 | 0.9999 | 0.39 | 0.98 |
| Fura | y = 1.65 × 106x + 1.07 × 104 | 0.137–6.85 | 0.9996 | 0.90 | 2.34 |
| Analytes | Precision | Stability | Repeatability | Accuracy | |||
|---|---|---|---|---|---|---|---|
| Intra-day RSD/% | Inter-day RSD/% | 18 h RSD/% | Concentration (mg∙g−1) | RSD/% | Recovery/% | RSD/% | |
| Zedo | 0.70 | 1.8 | 0.66 | 1.061 | 0.68 | 100.9 | 3.3 |
| Isoz | 0.51 | 1.7 | 0.81 | 0.396 | 0.90 | 98.4 | 3.7 |
| Aeru | 0.61 | 2.0 | 0.82 | 0.292 | 0.76 | 96.8 | 1.8 |
| Epox | 0.58 | 0.59 | 1.0 | 4.838 | 0.81 | 98.1 | 1.5 |
| Curc | 0.58 | 0.86 | 0.66 | 0.783 | 1.0 | 100.6 | 2.5 |
| Neoc | 0.48 | 1.5 | 0.48 | 1.843 | 0.68 | 99.2 | 2.9 |
| Curd | 1.8 | 1.4 | 1.7 | 9.875 | 0.72 | 97.8 | 1.8 |
| Germ | 1.0 | 0.70 | 1.4 | 2.720 | 1.0 | 96.2 | 2.5 |
| Fura | 0.66 | 1.3 | 1.3 | 7.618 | 1.0 | 100.9 | 2.2 |
| Analytes | 256 nm a | 244 nm b | 214 nm c | |||
|---|---|---|---|---|---|---|
| RRF | RSD/% | RRF | RSD/% | RRF | RSD/% | |
| Zedo | 0.022 | 4.5 | 0.249 | 2.4 | 3.698 | 0.89 |
| Isoz | 0.024 | 3.8 | 0.246 | 0.48 | 5.976 | 8.2 |
| Aeru | 0.015 | 3.0 | 0.131 | 1.0 | 0.612 | 6.2 |
| Epox | 0.039 | 4.3 | 0.226 | 2.0 | 0.904 | 0.49 |
| Curc | 0.015 | 4.4 | 0.147 | 1.8 | 2.289 | 4.5 |
| Neoc | 1.089 | 4.4 | 1.105 | 2.8 | 1.034 | 3.7 |
| Curd | 1.000 | 0.00 | 1.000 | 0.00 | 1.000 | 0.00 |
| Germ | 0.047 | 4.7 | 0.297 | 2.0 | 0.249 | 0.46 |
| Fura | 1.697 | 13.0 | 1.301 | 3.4 | 0.289 | 3.2 |
| Analytes | 256 nm a | 244 nm b | 214 nm c | |||||
|---|---|---|---|---|---|---|---|---|
| RRF | RSD/% | RRF | RSD/% | RRF | RSD/% | |||
| Zedo | 1.000 | 0.00 | 1.000 | 0.00 | 1.000 | 0.00 | ||
| Isoz | 1.085 | 1.7 | 0.986 | 2.0 | 1.626 | 8.2 | ||
| Aeru | 0.695 | 2.7 | 0.528 | 1.6 | 0.165 | 5.3 | ||
| Epox | 1.769 | 0.26 | 0.906 | 0.45 | 0.245 | 1.4 | ||
| Curc | 0.686 | 0.20 | 0.591 | 0.68 | 0.619 | 3.6 | ||
| Neoc | 48.935 | 9.4 | 4.328 | 4.2 | 0.287 | 5.6 | ||
| Curd | 45.192 | 4.5 | 4.017 | 2.5 | 0.270 | 0.89 | ||
| Germ | 2.133 | 0.49 | 1.194 | 0.41 | 0.067 | 1.3 | ||
| Fura | 78.789 | 15.4 | 5.345 | 4.7 | 0.078 | 2.4 | ||
| 256 nm | 244 nm | 214 nm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zedo | Curd | Zedo | Curd | Zedo | Curd | |||||||
| RRF a | RSD/% b | RRF | RSD/% | RRF | RSD/% | RRF | RSD/% | RRF | RSD/% | RRF | RSD/% | |
| Zedo | 1.000 | 0.00 | 0.022 | 10.5 | 1.000 | 0.00 | 0.253 | 4.2 | 1.000 | 0.00 | 3.697 | 0.93 |
| Isoz | 1.092 | 1.6 | 0.024 | 9.0 | 0.993 | 1.9 | 0.251 | 3.5 | 1.665 | 9.3 | 6.146 | 12.1 |
| Aeru | 0.696 | 2.6 | 0.015 | 8.3 | 0.524 | 2.5 | 0.132 | 2.3 | 0.163 | 2.9 | 0.603 | 2.9 |
| Epox | 1.795 | 1.6 | 0.040 | 9.9 | 0.914 | 1.9 | 0.230 | 3.8 | 0.246 | 0.54 | 0.910 | 0.74 |
| Curc | 0.693 | 2.3 | 0.015 | 9.1 | 0.604 | 3.9 | 0.150 | 3.0 | 0.617 | 3.7 | 2.239 | 1.5 |
| Neoc | 51.195 | 22.5 | 1.138 | 16.3 | 4.299 | 6.0 | 1.092 | 3.2 | 0.277 | 4.6 | 1.016 | 4.6 |
| Curd | 44.187 | 8.5 | 1.000 | 0.00 | 3.969 | 5.0 | 1.000 | 0.00 | 0.272 | 0.49 | 1.000 | 0.00 |
| Germ | 2.161 | 1.9 | 0.048 | 9.0 | 1.217 | 3.3 | 0.305 | 3.1 | 0.067 | 1.8 | 0.249 | 1.1 |
| Fura | 84.812 | 10.1 | 1.679 | 18.3 | 5.451 | 14.3 | 1.309 | 4.5 | 0.077 | 3.1 | 0.286 | 2.4 |
| Samples | Zedo | Isoz | Aeru | Epox | Curc | Neoc | Curd | Germ | Fura | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 b | 2 c | RE/% d | 1 | 2 | RE/% | 1 | 2 | RE/% | 1 | 2 | RE/% | 1 | 2 | RE/% | 1 | 2 | RE/% | 2 | 1 | 2 | RE/% | 1 | 2 | RE/% | |
| PJH-1 a | 1.02 | 0.968 | 5.1 | 0.405 | 0.397 | 2.0 | 0.281 | 0.277 | 1.4 | 6.07 | 5.95 | 2.0 | 0.874 | 0.865 | 1.0 | 2.07 | 2.03 | 1.9 | 10.82 | 2.44 | 2.42 | 0.82 | 8.32 | 8.17 | 1.8 |
| PJH-2 | 1.63 | 1.55 | 4.9 | 0.664 | 0.648 | 2.4 | 0.423 | 0.418 | 1.2 | 11.25 | 11.02 | 2.0 | 1.26 | 1.24 | 1.6 | 3.26 | 3.18 | 2.5 | 16.93 | 5.20 | 5.14 | 1.2 | 15.9 | 15.60 | 1.9 |
| PJH-3 | 0.767 | 0.729 | 5.0 | 0.286 | 0.281 | 1.8 | 0.178 | 0.177 | 0.56 | 3.90 | 3.83 | 1.8 | 0.619 | 0.614 | 0.81 | 1.43 | 1.41 | 1.4 | 6.93 | 1.64 | 1.63 | 0.61 | 5.56 | 5.48 | 1.4 |
| PJH-4 | 1.35 | 1.29 | 4.4 | 0.527 | 0.515 | 2.3 | 0.456 | 0.450 | 1.3 | 7.92 | 7.75 | 2.1 | 1.22 | 1.20 | 1.6 | 2.90 | 2.82 | 2.8 | 14.33 | 4.07 | 4.03 | 0.98 | 12.57 | 12.33 | 1.9 |
| PJH-5 | 1.08 | 1.03 | 4.6 | 0.421 | 0.412 | 2.1 | 0.347 | 0.343 | 1.2 | 6.04 | 5.92 | 2.0 | 0.898 | 0.888 | 1.1 | 2.05 | 2.01 | 2.0 | 10.55 | 3.00 | 2.98 | 0.67 | 8.24 | 8.10 | 1.7 |
| WEZ-1 | 0.754 | 0.717 | 4.9 | 0.284 | 0.280 | 1.4 | 0.181 | 0.180 | 0.55 | 0.119 | 0.123 | 3.4 | 3.44 | 3.38 | 1.7 | 1.65 | 1.62 | 1.8 | 8.78 | 2.41 | 2.39 | 0.83 | 7.56 | 7.43 | 1.7 |
| WEZ-2 | 0.453 | 0.432 | 4.6 | 0.160 | 0.159 | 0.63 | 0.085 | 0.086 | 1.2 | 0.088 | 0.093 | 5.7 | 2.07 | 2.04 | 1.4 | 1.16 | 1.15 | 0.86 | 5.55 | 1.36 | 1.35 | 0.74 | 3.64 | 3.61 | 0.82 |
| WEZ-3 | 0.668 | 0.635 | 4.9 | 0.239 | 0.236 | 1.3 | 0.135 | 0.134 | 0.74 | 0.223 | 0.225 | 0.90 | 3.55 | 3.49 | 1.7 | 1.68 | 1.65 | 1.8 | 9.73 | 2.43 | 2.41 | 0.82 | 6.77 | 6.66 | 1.6 |
| WEZ-4 | 0.826 | 0.784 | 5.1 | 0.313 | 0.308 | 1.6 | 0.160 | 0.159 | 0.63 | 0.226 | 0.228 | 0.88 | 4.41 | 4.33 | 1.8 | 1.84 | 1.80 | 2.2 | 9.98 | 2.67 | 2.65 | 0.75 | 6.24 | 6.15 | 1.4 |
| WEZ-5 | 0.638 | 0.606 | 5.0 | 0.233 | 0.230 | 1.3 | 0.191 | 0.190 | 0.52 | 1.75 | 1.72 | 1.7 | 1.82 | 1.79 | 1.6 | 1.90 | 1.86 | 2.1 | 9.21 | 2.80 | 2.78 | 0.71 | 9.20 | 9.04 | 1.7 |
| WYJ-1 | 0.133 | 0.128 | 3.8 | 0.057 | 0.059 | 3.5 | – | – | – | – | – | – | 0.479 | 0.477 | 0.42 | 0.296 | 0.314 | 6.1 | 1.15 | 0.256 | 0.261 | 2.0 | 0.718 | 0.753 | 4.9 |
| WYJ-2 | 0.150 | 0.145 | 3.3 | 0.068 | 0.070 | 2.9 | – | – | – | – | – | – | 0.216 | 0.219 | 1.4 | 0.472 | 0.484 | 2.5 | 0.559 | – | – | – | – | – | – |
| WYJ-3 | 0.158 | 0.152 | 3.8 | 0.068 | 0.070 | 2.9 | – | – | – | – | – | – | 0.744 | 0.737 | 0.94 | 0.352 | 0.368 | 4.5 | 1.88 | 0.580 | 0.581 | 0.17 | 1.59 | 1.61 | 1.3 |
| WYJ-4 | 0.148 | 0.143 | 3.4 | 0.058 | 0.060 | 3.5 | 0.022 | 0.023 | 4.5 | 0.076 | 0.082 | 7.9 | 0.698 | 0.692 | 0.86 | 0.267 | 0.286 | 7.1 | 2.09 | 0.818 | 0.816 | 0.24 | 2.00 | 2.01 | 0.50 |
| WYJ-5 | 0.265 | 0.254 | 4.2 | 0.105 | 0.105 | 0.00 | 0.051 | 0.052 | 2.0 | 0.093 | 0.098 | 5.4 | 0.393 | 0.393 | 0.00 | 0.277 | 0.296 | 6.9 | 2.02 | 0.711 | 0.711 | 0.00 | 1.44 | 1.46 | 1.4 |
| EO-1 | – e | – | – | – | – | – | – | – | – | 0.672 | 0.665 | 1.0 | 16.48 | 16.24 | 1.5 | 19.66 | 18.94 | 3.7 | 217.4 | 93.77 | 92.72 | 1.1 | 100.3 | 103.9 | 3.6 |
| EO-2 | – | – | – | – | – | – | – | – | – | – | – | – | 9.53 | 9.39 | 1.5 | 21.59 | 20.80 | 3.7 | 238.2 | 104.6 | 103.4 | 1.1 | 115.5 | 119.6 | 3.5 |
| EO-3 | – | – | – | – | – | – | – | – | – | 0.323 | 0.319 | 1.2 | 5.41 | 5.33 | 1.5 | 12.90 | 12.42 | 3.7 | 245.32 | 134.7 | 133.2 | 1.1 | 123.4 | 127.7 | 3.5 |
| FFEZY-1 | – | – | – | – | – | – | – | – | – | 0.087 | 0.087 | 0.00 | 1.10 | 1.08 | 1.8 | 1.51 | 1.45 | 4.0 | 12.75 | 6.45 | 6.38 | 1.1 | 10.91 | 11.29 | 3.5 |
| FFEZY-2 | – | – | – | – | – | – | – | – | – | 0.074 | 0.074 | 0.00 | 1.09 | 1.07 | 1.8 | 1.53 | 1.48 | 3.3 | 12.57 | 5.72 | 5.66 | 1.0 | 7.02 | 7.26 | 3.4 |
| FFEZY-3 | – | – | – | – | – | – | – | – | – | 0.470 | 0.465 | 1.1 | 0.976 | 0.962 | 1.4 | 2.51 | 2.41 | 4.0 | 19.99 | 7.93 | 7.85 | 1.0 | 7.04 | 7.29 | 3.6 |
© 2013 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, J.-J.; An, Y.-W.; Hu, G.; Yin, G.-P.; Zhang, Q.-W.; Wang, Z.-M. Simultaneous Determination of Multiple Sesquiterpenes in Curcuma wenyujin Herbal Medicines and Related Products with One Single Reference Standard. Molecules 2013, 18, 2110-2121. https://doi.org/10.3390/molecules18022110
Zhu J-J, An Y-W, Hu G, Yin G-P, Zhang Q-W, Wang Z-M. Simultaneous Determination of Multiple Sesquiterpenes in Curcuma wenyujin Herbal Medicines and Related Products with One Single Reference Standard. Molecules. 2013; 18(2):2110-2121. https://doi.org/10.3390/molecules18022110
Chicago/Turabian StyleZhu, Jing-Jing, Yue-Wei An, Guang Hu, Guo-Ping Yin, Qi-Wei Zhang, and Zhi-Min Wang. 2013. "Simultaneous Determination of Multiple Sesquiterpenes in Curcuma wenyujin Herbal Medicines and Related Products with One Single Reference Standard" Molecules 18, no. 2: 2110-2121. https://doi.org/10.3390/molecules18022110
APA StyleZhu, J. -J., An, Y. -W., Hu, G., Yin, G. -P., Zhang, Q. -W., & Wang, Z. -M. (2013). Simultaneous Determination of Multiple Sesquiterpenes in Curcuma wenyujin Herbal Medicines and Related Products with One Single Reference Standard. Molecules, 18(2), 2110-2121. https://doi.org/10.3390/molecules18022110