3-Substituted Prolines: From Synthesis to Structural Applications, from Peptides to Foldamers †
Abstract
:1. Introduction


2. Syntheses
2.1. Syntheses Starting from Proline or Proline Derivatives








2.2. Syntheses by Intramolecular Cyclization Processes
2.2.1. C–C Bond Formation through Anionic Processes


2.2.2. C–C Bond Formation through Cationic Process

2.2.3. C–C Bond Formation through Radicalar Process

2.2.4. C–N Bond Formation
2.2.4.1. From Aspartic or Glutamic Acid Derivatives

2.2.4.2. From Garner’s Aldehyde


2.2.4.3. Through Reductive Amination

2.3. Synthesis of 3-Substituted Prolines by Intermolecular Cyclization
2.3.1. Through Michael-Addition/Alkylation Sequences

3. Conformational Effects and Structural Applications
3.1. Conformational Effects of 3-Substituted Prolines

3.2. Structural Applications
3.2.1. Polyproline Helical Conformations


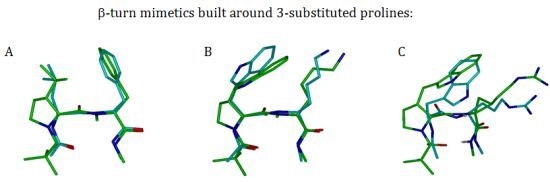
3.2.2. β-Turns

4. Conclusions
Acknowledgments
References and Notes
- Wawra, S.; Fischer, G. Amide cis-trans Isomerisation in Peptides and Proteins. In Cis-Trans Isomerization in Biochemistry; Dugave, E.C., Ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 167. [Google Scholar]
- Aubry, A.; Vitoux, B.; Marraud, M. Conformational properties of Pro–Pro sequences. I. Crystal structures of two dipeptides with L-Pro-L-Pro and L-Pro-D-Pro sequences. Biopolymers 1985, 24, 1089–1100. [Google Scholar] [CrossRef]
- Fillon, Y.A.; Anderson, J.P.; Chmielewski, J. Cell Penetrating agents based on a polyproline helix scaffold. J. Am. Chem. Soc. 2005, 127, 11798–11803. [Google Scholar] [CrossRef]
- Jacquot, Y.; Broutin, I.; Miclet, E.; Nicaise, M.; Lequin, O.; Goasdoué, N.; Joss, C.; Karoyan, P.; Desmadril, M.; Ducruix, A.; Lavielle, S. High affinity Grb2-SH3 domain ligand incorporating Cβ-substituted prolines in a Sos-derived decapeptide. Bioorg. Med. Chem. 2007, 15, 1439–1447. [Google Scholar]
- Karoyan, P.; Sagan, S.; Lequin, O.; Quancard, J.; Lavielle, S.; Chassaing, G. Susbtituted Prolines: Syntheses and Applications in Structure-Activity Relationship Studies of Biologically Active Peptides. In Targets in Heterocyclic Systems-Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2004; Volume 8, pp. 216–273. [Google Scholar]
- Huck, B.R.; Fisk, J.D.; Guzei, I.A.; Carlson, H.A.; Gellman, S.H. Secondary structural preferences of 2,2-disubstituted pyrrolidine-4-carboxylic acid oligomers: β-Peptide foldamers that cannot form internal hydrogen bonds. J. Am Chem. Soc. 2003, 125, 9035–9037. [Google Scholar]
- Huck, B.R.; Gellman, S.H. Synthesis of 2,2-disubstituted pyrrolidine-4-carboxylic acid derivatives and their incorporation into β-peptide oligomers. J. Org. Chem. 2005, 70, 3353–3362. [Google Scholar] [CrossRef]
- Medda, A.K.; Lee, H.-S. 3,4-Methano-β-proline: A conformationally constrained β-amino acid. Synlett 2009, 921–924. [Google Scholar]
- Krow, G.R.; Liu, N.; Sender, M.; Lin, G.; Centafont, R.; Sonnet, P.E.; DeBrosse, C.; Ross, C.W.; Carroll, P.J.; Shoulders, M.D.; Raines, R.T. Oligomers of a 5-carboxy-methanopyrrolidine-amino acid. A search for order. Org. Lett. 2010, 12, 5438–5441. [Google Scholar]
- Otani, Y.; Futaki, S.; Kiwada, T.; Sugiura, Y.; Muranaka, A.; Kobayashi, N.; Uchiyama, M.; Yamaguchi, K.; Ohwada, T. Oligomers of β-amino acid bearing non-planar amides form ordered structures. Tetrahedron 2006, 62, 11635–11644. [Google Scholar]
- Hosoya, M.; Otani, Y.; Kawahata, M.; Yamaguchi, K.; Ohwada, T. Water-stable helical structure of tertiary amides of bicyclic -amino acid bearing 7-azabicyclo[2.2.1]heptane. Full control of amide cis-trans equilibrium by bridgehead substitution. J. Am. Chem. Soc. 2010, 132, 14780–14789. [Google Scholar] [CrossRef]
- Abele, S.; Vögtli, K.; Seebach, D. Oligomers of β2- and of β3-Homoproline: What are the secondary structures of β-peptides lacking H-Bonds? Helv. Chim. Acta 1999, 82, 1539–1558. [Google Scholar] [CrossRef]
- Häusler, J.; Schmidt, U. Synthese von cis- und trans-3-Phenoxyprolin. Liebigs Ann. Chem. 1979, 1881–1889. [Google Scholar] [CrossRef]
- Häusler, J. Darstellung von cis- und trans-C-3-substituierten Prolinverbindungen. Liebigs Ann. Chem. 1981, 1073–1088. [Google Scholar] [CrossRef]
- Mothes, C. Prolino Amino Acides Substitués en Position 3. Synthèses, Applications Structurales et Pharmacologiques dans le Développement D'inhibiteurs D'interactions Peptide-Protéine et Protéine-Protéine. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France.
- Huy, P.; Neudörfl, J.-M.; Schmalz, H.-G. A practical synthesis of trans-3-substituted proline derivatives through 1,4-addition. Org. Lett. 2011, 13, 216–219. [Google Scholar] [CrossRef]
- Maillard, M.C.; Brookfield, F.A.; Courtney, S.M.; Eustache, F.M.; Gemkow, M.J.; Handel, R.K.; Johnson, L.C.; Johnson, P.D.; Kerry, M.A.; Krieger, F.; et al. Exploiting differences in caspase-2 and -3 S2 subsites for selectivity: Structure-based design, solid-phase synthesis and in vitro activity of novel substrate-based caspase-2 inhibitors. Bioorg. Med. Chem. 2011, 19, 5833–5851. [Google Scholar]
- Moss, W.O.; Jones, A.C.; Wisedale, R.; Mahon, M.F.; Molloy, K.C.; Bradbury, R.H.; Hales, N.J.; Gallagher, T. 2-Amino ketene S,S-acetals as a-amino acid homoenolate equivalents. Synthesis of 3-substituted prolines and molecular structure of 2-(N-pivaloylpyrrolidin-2-ylidene)-1.3-dithiane. J. Chem. Soc. Perkin Trans. 1 1992, 2615–2624. [Google Scholar]
- Holladay, M.W.; Lin, C.W.; May, C.S.; Garvey, D.S.; Witte, D.G.; Miller, T.R.; Wolfram, C.A.W.; Nadzan, A.M. trans-3-n-Propyl-L-proline is a highly favorable, conformationally restricted replacement for methionine in the C-terminal tetrapeptide of cholecystokinin. Stereoselective synthesis of 3-allyl- and 3-n-propyl-l-proline derivatives from 4-hydroxy-l-proline. J. Med. Chem. 1991, 34, 455–457. [Google Scholar] [CrossRef]
- Sharma, R.; Lubell, W.D. Regioselective enolization and alkylation of 4-oxo-N-(9-phenylfluoren-9-yl)proline: Synthesis of enantiopure proline−valine and hydroxyproline−valine chimeras. J. Org. Chem. 1996, 61, 202–209. [Google Scholar] [CrossRef]
- Mamai, A.; Hughes, N.E.; Wurthmann, A.; Madalengoitia, J.S. Synthesis of conformationally constrained arginine and ornithine analogues based on the 3-substituted pyrrolidine framework. J. Org. Chem. 2001, 66, 6483–6486. [Google Scholar]
- Kamenecka, T.M.; Park, Y.-J.; Lin, L.S.; Lanza, T.J.; Hagmann, W.K. Enantioselective approach to 3-substituted prolines. Tetrahedron Lett. 2001, 42, 8571–8573. [Google Scholar] [CrossRef]
- Pellegrini, N.; Schmitt, M.; Guery, S.; Bourgignon, J.-J. New strategies towards proline derivatives as conformationally constrained arginine analogues. Tetrahedron Lett. 2002, 43, 3243–3246. [Google Scholar] [CrossRef]
- Karoyan, P.; Chassaing, G. New strategy for the synthesis of 3-substituted prolines. Tetrahedron Lett. 1997, 38, 85. [Google Scholar] [CrossRef]
- Lorthiois, E.; Marek, I.; Normant, J.-F. Zinca-ene-allene and zinc-enolate cyclization. Towards the synthesis of polysubstituted pyrrolidines. Tetrahedron Lett. 1997, 38, 89–92. [Google Scholar] [CrossRef]
- Karoyan, P.; Quancard, J.; Vaissermann, J.; Chassaing, G. Amino-Zinc-Enolate carbometalation reactions: Application to ring closure of terminally substituted olefin for the asymmetric synthesis of cis- and trans-3-prolinoleucine. J. Org. Chem. 2003, 68, 2256–2265. [Google Scholar] [CrossRef]
- Quancard, J.; labonne, A.; Jacquot, Y.; Lavielle, S.; Chassaing, G.; Karoyan, P. Asymmetric synthesis of 3-substituted proline chimeras bearing polar side chains of proteinogenic amino acids. J. Org. Chem. 2004, 69, 7940. [Google Scholar] [CrossRef]
- Mothes, C.; Lavielle, S.; Karoyan, P. Amino-zinc-ene-enolate cyclization: A short access to cis-3-substituted prolino-homotryptophane derivatives. 2008. [Google Scholar]
- Amine commercially available from Genzyme, Eichenweg 1, CH-4410 Liestal Switzerland, Tel.: +41-(0)61-906-5959 Fax: +41-(0)61-906-5958.
- Mooiver, H.H.; Hiemstra, H.; Fortgens, H.P.; Speckamp, W.N. Intramolecular reactions of acyclic n-acyliminium ions III silicon assisted cyclocondensation of glyoxylic esters to proline and pipecolic acid derivatives. Tetrahedron Lett. 1987, 28, 3285–3288. [Google Scholar] [CrossRef]
- Esch, P.M.; Hiemstra, H.; Speckamp, W.N. Synthesis of cyclic α-amino acids through ring closure of glycine derived free radicals. Tetrahedron Lett. 1990, 31, 759–762. [Google Scholar] [CrossRef]
- Udding, J.H.; Giesselink, J.P.M.; Hiemstra, H.; Speckamp, W.N. Xanthate transfer cyclisation of glycine radicals; synthesis of 5- and 6-membered ring nitrogen heterocycles. Bull. Soc. Chim. Belg. 1994, 103, 329–342. [Google Scholar]
- Udding, J. H.; Tuijp, J.M.; Hiemstra, H.; Speckamp, W.N. Transition metal-catalyzed chlorine transfer cyclizations of carbon-centered glycine radicals; a novel synthesis route to cyclic α-amino acids. Tetrahedron 1994, 35, 1907–1918. [Google Scholar]
- Cotton, R.; Johnstone, A.N.C.; North, M. Asymmetric synthesis of 3-carboxyproline and derivatives suitable for peptide synthesis. Tetrahedron 1995, 51, 8525–8544. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Moloney, M.G.; North, M. Asymmetric amino acid synthesis: preparation of the β anion derived from aspartic acid. J. Chem. Soc. Perkin Trans. 1 1989, 833–834. [Google Scholar] [CrossRef]
- Flamant-Robin, C.; Wang, Q.; Chiaroni, A.; Sasaki, A. An efficient method for the stereoselective synthesis of cis-3-substituted prolines: Conformationally constrained α-amino acids. Tetrahedron 2002, 58, 10475–10484. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, Q.; Sasaki, A. Synthesis of conformationally constrained analogues of RGD tripeptide. Tetrahedron 2007, 63, 2084–2092. [Google Scholar] [CrossRef]
- Sasaki, A.; Hashimoto, C.; Chiaroni, A.; Riche, C.; Potier, P. A novel approach to the synthesis of optically pure non protein α-amino acids in both L and D configurations from L-serine. Tetrahedron Lett. 1987, 28, 6069–6072. [Google Scholar] [CrossRef]
- Sasaki, A.; Dockner, M.; Chiaroni, A.; Riche, C.; Potier, P. A novel stereodivergent synthesis of optically pure cis- and trans-3-substituted proline derivatives. J. Org. Chem. 1997, 62, 765–770. [Google Scholar] [CrossRef]
- Sasaki, A.; Pauly, R.; Fontaine, C.; Chiaroni, A.; Riche, C.; Potier, P. Enantioselective synthesis of (2S,3S)- and (2R,3R)-pyrrolidine-2,3-dicarboxylic acids: Conformationally constrained (S)- and (R)-aspartic acid analogues. Tetrahedron Lett. 1994, 35, 241–244. [Google Scholar]
- Han, M.-Y.; Zhang, Y.; Wang, H.-Z.; An, W.-K.; Ma, B.-C.; Zhang, Y.; Wanga, W. Organocatalytic michael addition of nitro esters to a,b-unsaturated aldehydes: Towards the enantioselective synthesis of trans-3-substituted proline derivatives. Adv. Synth. Catal. 2012, 354, 2635–2640. [Google Scholar] [CrossRef]
- Cox, D.A.; Johnson, A.W.; Mauger, A.B. A modified proline synthesis. J. Chem. Soc. 1964, 5024–5029. [Google Scholar]
- Mosberg, H.I.; Omnaas, J.R.; Lomize, A.; Heyl, D.L.; Nordan, I.; Mousigian, C.; Davis, P.; Porreca, F. Development of a model for the δ opioid receptor pharmacophore. 1. Conformationally restricted Tyr1 replacements in the cyclic δ receptor selective tetrapeptide Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13). J. Med. Chem. 1994, 37, 4371–4383. [Google Scholar] [CrossRef]
- Chung, J.Y.L.; Wasicak, J.T.; Arnold, W.A.; May, C.S.; Nadzan, A.M.; Holladay, M.W. Conformationally constrained amino acids. Synthesis and optical resolution of 3-substituted proline derivatives. J. Org. Chem. 1990, 55, 270–275. [Google Scholar] [CrossRef]
- Damour, D.; Doerflinger, G.; Pantel, G.; Labaudinière, R.; Leconte, J.-P.; Sablé, S.; Vuilhorgne, M.; Mignani, S. A convenient synthetic route to macrocyclic cis-3-phenylproline derivatives as mimics of sandostatin®. Synlett 1999, 189–192. [Google Scholar]
- Rios, R.; Ibrahem, I.; Vesely, J.; Sundén, H.; Cordova, A. Organocatalytic asymmetric 5-hydroxypyrrolidine synthesis: A highly enantioselective route to 3-substituted proline derivatives. Tetrahedron Lett. 2007, 48, 8695–8699. [Google Scholar] [CrossRef]
- Delaney, N.G.; Madison, V. Novel conformational distributions of methylproline peptides. J. Am. Chem. Soc. 1982, 104, 6635–6641. [Google Scholar] [CrossRef]
- Quancard, J.; Karoyan, P.; Lequin, O.; Wenger, E.; Aubry, A.; Lavielle, S.; Chassaing, G. Prolinoamino acids as a tool to stabilize β-turns with the side chain of natural amino acids. Tetrahedron Lett. 2004, 45, 623–625. [Google Scholar]
- Beausoleil, E.; Sharma, R.; Michnick, S.W.; Lubell, W.D. Alkyl 3-position substituents retard the isomerization of prolyl and hydroxyprolyl amides in water. J. Org. Chem. 1998, 63, 6572–6578. [Google Scholar] [CrossRef]
- Mothes, C.; Larregola, M.; Quancard, J.; Goasdoué, N.; Lavielle, S.; Chassaing, G.; Lequin, O.; Karoyan, P. Prolinoamino acids as tools to build bifunctionalized, stable β-turns in water. ChemBioChem. 2010, 11, 55–58. [Google Scholar]
- Brodsky, B.; Thiagarajan, G.; Madhan, B.; Kar, K. Triple-helical peptides: An approach to collagen conformation, stability, and self-association. Biopolymers 2008, 89, 345–353. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Rabanal, F.; Ludevid, M.D.; Pons, M.; Giralt, E. CD the of proline-rich polypeptides: Application to the study of repetitive domain of maize glutelin-2. Biopolymers 1993, 33, 1019–1028. [Google Scholar] [CrossRef]
- Kay, B.K.; Williamson, M.P.; Sudol, M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000, 14, 231–241. [Google Scholar]
- Williamson, M.P. The structure and function of proline-rich regions in proteins. Biochem. J. 1994, 297, 249–260. [Google Scholar]
- Kalafut, D.; Anderson, T.N.; Chmielewski, J. Mitochondrial targeting of a cationic amphiphilic polyproline helix. Bioorg. Med. Chem. Lett. 2012, 22, 561–563. [Google Scholar] [CrossRef]
- Caumes, C.; Delsuc, N.; Beni Azza, R.; Correia, I.; Chemla, F.; Ferreira, F.; Carlier, L.; Perez Luna, A.; Moumné, R.; Lequin, O.; et al. Homooligomers of substituted proline and β-prolines: Synthesis and secondary structure investigation by CD experiments. New J. Chem. 2013. submitted for publication. [Google Scholar]
- Devos, A.; Remion, J.; Frisque-Hesbain, A.-M.; Colens, A.; Ghosez, L. Synthesis of acyl halides under very mild conditions. J. Chem. Soc. Chem. Commun. 1979, 1180–1181. [Google Scholar]
- Kümin, M.; Sonntag, L.-S.; Wennemers, H. Azidoproline containing helices: Stabilization of the polyproline II structure by a functionalizable group. J. Am. Chem. Soc. 2007, 129, 466–467. [Google Scholar] [CrossRef]
- Sonar, M.V.; Ganesh, K.N. Water-induced switching of β-structure to polyproline II conformation in the 4S-aminoproline polypeptide via H-bond rearrangement. Org. Lett. 2010, 12, 5390–5393. [Google Scholar] [CrossRef]
- Zhang, R.; Brownewell, F.; Madalengoitia, J.S. Pseudo-A(1,3) Strain as a key conformational control element in the design of poly-L-proline type II peptide mimics. J. Am. Chem. Soc. 1998, 120, 3894–3902. [Google Scholar] [CrossRef]
- Kuemin, M.; Nagel, Y.A.; Schweizer, S.; Monnard, F.W.; Ochsenfeld, C.; Wennemers, H. Tuning the cis/trans conformer ratio of Xaa–Pro amide bonds by intramolecular hydrogen bonds: The effect on PPII helix stability. Angew. Chem. Int. Ed. 2010, 49, 6324–6327. [Google Scholar] [CrossRef]
- McCafferty, D.G.; Friesen, D.A.; Danielson, E.; Wall, C.G.; Saderholm, M.J.; Erickson, B.W.; Meyer, T.J. Photochemical energy conversion in a helical oligoproline assembly. Proc. Natl. Acad. Sci. USA 1996, 93, 8200–8204. [Google Scholar]
- Sagan, S.; Quancard, J.; Lequin, O.; Karoyan, P.; Chassaing, G.; Lavielle, S. Conformational analysis of the C-Terminal Gly-Leu-Met-NH2 tripeptide of substance P bound to the NK-1 receptor. Chem. Biol. 2005, 12, 555–565. [Google Scholar] [CrossRef]
- Tyndall, J.D.; Pfeiffer, B.; Abbenante, G.; Fairlie, D.P. Over one hundred peptide-activated G protein-coupled receptors recognize ligands with turn structure. Chem. Rev. 2005, 105, 793–826. [Google Scholar] [CrossRef]
- Seebach, D.; Abele, S.; Gademann, K.; Jaun, B. Pleated sheets and turns of β-peptides with proteinogenic side chains. Angew. Chem. Int. Ed. 1999, 38, 1595–1597. [Google Scholar] [CrossRef]
- Gademann, K.; Kimmerlin, T.; Hoyer, D.; Seebach, D. Peptide folding induces high and selective affinity of a linear and small β-peptide to the human somatostatin receptor 4. J. Med. Chem. 2001, 44, 2460–2468. [Google Scholar] [CrossRef]
- Guitot, K.; Larregola, M.; Pradhan, T.K.; Vasse, J.-L.; Lavielle, S.; Bertus, P.; Szymoniak, J.; Lequin, O.; Karoyan, P. The combination of prolinoamino acids and cyclopropylamino acids leads to fully functionalized, stable β-turns in water. ChemBioChem 2011, 12, 1039–1042. [Google Scholar] [CrossRef]
- Ball, J.B.; Alewood, P.F. Conformational constraints: Nonpeptide β-turn mimics. J. Mol. Recognit. 1990, 3, 55–64. [Google Scholar] [CrossRef]
- Souers, A.J.; Ellman, J.A. β-Turn mimetic library synthesis: Scaffolds and applications. Tetrahedron 2001, 57, 7431–7448. [Google Scholar] [CrossRef]
- Kee, K.S.; Jois, S.D.S. Design of β-turn based therapeutic agents. Curr. Pharm. Des. 2003, 9, 1209–1224. [Google Scholar] [CrossRef]
- Barbaras, D.; Gademann, K. Stable β turns of tripeptides in water through cation–π interactions. ChemBioChem 2008, 9, 2398–2401. [Google Scholar] [CrossRef]
- Imperiali, B.; Moats, R.A.; Fisher, S.L.; Prins, T.J. A conformational study of peptides with the general structure Ac-L-Xaa-Pro-D-Xaa-L-Xaa-NH2: Spectroscopic evidence for a peptide with significant β-turn character in water and in dimethyl sulfoxide. J. Am. Chem. Soc. 1992, 114, 3182–3188. [Google Scholar]
- Chalmers, D.K.; Marshall, G.M. Pro-D-NMe-Amino Acid and D-Pro-NMe-Amino Acid: Simple, efficient reverse-turn constraints. J. Am. Chem. Soc. 1995, 117, 5927–5937. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Marshall, G.M. Conformational analysis of reverse-turn constraints by N-methylation and N-hydroxylation of amide bonds in peptides and non-peptide mimetics. J. Am. Chem. Soc. 1998, 120, 5363–5372. [Google Scholar] [CrossRef]
- Chatterjee, B.; Saha, I.; Raghothama, S.; Aravinda, S.; Rai, R.; Shamala, N.; Balaram, P. Designed peptides with homochiral and heterochiral diproline templates as conformational constraints. Chem. Eur. J. 2008, 14, 6192–6204. [Google Scholar]
- Weckbecker, G.; Lewis, I.; Albert, R.; Schmid, H.A.; Hoyer, D.; Bruns, C. Opportunities in somatostatin research: Biological, chemical and therapeutic aspects. Nat. Rev. Drug Discov. 2003, 2, 999–1017. [Google Scholar] [CrossRef]
- Wiegand, G.; Epp, O.; Huber, R. The crystal structure of porcine pancreatic α-amylase in complex with the microbial inhibitor tendamistat. J. Mol. Biol. 1995, 247, 99–110. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mothes, C.; Caumes, C.; Guez, A.; Boullet, H.; Gendrineau, T.; Darses, S.; Delsuc, N.; Moumné, R.; Oswald, B.; Lequin, O.; et al. 3-Substituted Prolines: From Synthesis to Structural Applications, from Peptides to Foldamers. Molecules 2013, 18, 2307-2327. https://doi.org/10.3390/molecules18022307
Mothes C, Caumes C, Guez A, Boullet H, Gendrineau T, Darses S, Delsuc N, Moumné R, Oswald B, Lequin O, et al. 3-Substituted Prolines: From Synthesis to Structural Applications, from Peptides to Foldamers. Molecules. 2013; 18(2):2307-2327. https://doi.org/10.3390/molecules18022307
Chicago/Turabian StyleMothes, Céline, Cécile Caumes, Alexandre Guez, Héloïse Boullet, Thomas Gendrineau, Sylvain Darses, Nicolas Delsuc, Roba Moumné, Benoit Oswald, Olivier Lequin, and et al. 2013. "3-Substituted Prolines: From Synthesis to Structural Applications, from Peptides to Foldamers" Molecules 18, no. 2: 2307-2327. https://doi.org/10.3390/molecules18022307
APA StyleMothes, C., Caumes, C., Guez, A., Boullet, H., Gendrineau, T., Darses, S., Delsuc, N., Moumné, R., Oswald, B., Lequin, O., & Karoyan, P. (2013). 3-Substituted Prolines: From Synthesis to Structural Applications, from Peptides to Foldamers. Molecules, 18(2), 2307-2327. https://doi.org/10.3390/molecules18022307