Applications of Biomaterials to Liquid Crystals
Abstract
:1. Introduction
2. What are Liquid Crystals?


3. New Functional LC Modulated with Peptide

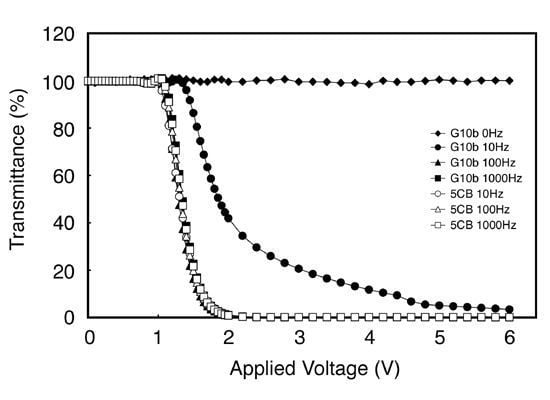
4. Liquid Crystals and Doping Agents

5. Biological Applications


6. Conclusions
Acknowledgments
References
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef]
- Cavalli, S.; Albericio, F.; Kros, A. Amphiphilic peptides and their cross-disciplinary role as building blocks for nanoscience. Chem. Soc. Rev. 2010, 39, 241–263. [Google Scholar] [CrossRef]
- Hamley, I.W. Self-assembly of amphiphilic peptides. Soft Matter 2011, 7, 4122–4138. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, F.; Xu, H.; Yaseen, M.; Shan, H.; Hause, C.A.E.; Zhang, S.; Lu, J.R. Molecular self-assembly and applications of designer peptide amphipholes. Chem. Soc. Rev. 2010, 39, 3480–3498. [Google Scholar] [CrossRef]
- Versluis, F.; Marsden, H.R.; Kros, A. Power struggles in peptide-amphiphile nanostructures. Chem. Soc. Rev. 2010, 39, 3434–3444. [Google Scholar] [CrossRef]
- Seeman, N.C. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef]
- Gothelf, K.V.; LaBean, T.H. DNA-programmed assembly of nanostructures. Org. Biomol. Chem. 2005, 3, 4023–4037. [Google Scholar] [CrossRef]
- Chworos, A.; Severcan, I.; Koyfman, A.Y.; Weinkam, P.; Oroudjev, E.; Hansma, H.G.; Jaeger, L. Building Programmable Jigsaw Puzzles with RNA. Science 2004, 306, 2068–2072. [Google Scholar] [CrossRef]
- Leontis, N.B.; Lescoute, A.; Westhof, E. The building blocks an motifs of RNA architecture. Curr. Opin. Struct. Biol. 2006, 16, 279–287. [Google Scholar] [CrossRef]
- Severcan, I.; Geary, C.; Verzemnieks, E.; Chworos, A.; Jaeger, L. Square-Shaped RNA Particles from Different RNA Folds. Nano Lett. 2009, 9, 1270–1277. [Google Scholar] [CrossRef]
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef]
- Löwik, D.W.P.M.; Leunissen, E.H.P.; van den Heuvel, M.; Hansen, M.B.; van Hest, J.C.M. Stimulus responsive peptide based materials. Chem. Soc. Rev. 2010, 39, 3394–3412. [Google Scholar] [CrossRef]
- Scheibel, T.; Parthasarathy, R.; Sawicki, G.; Lin, X.M.; Jaeger, H.; Lindquist, S.L. Conducting nanowires built by controlled self-assembly of amyloid fibers an selective metal deposition. Proc. Natl. Acad. Sci. USA 2003, 100, 4527–4532. [Google Scholar] [CrossRef]
- Del Mercato, L.L.; Pompa, P.P.; Maruccio, G.; Torre, A.D.; Sabella, S.; Tamburro, A.M.; Cingolani, R.; Rinaldi, R. Charge transport and intrinsic fluorescence in amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 2007, 104, 18019–18024. [Google Scholar] [CrossRef]
- Kumar, R.; MacDonald, J.M.; Singh, Th.B.; Waddington, L.J.; Holmes, A.B. Hierarchical Self-assembly of semiconductor functionalized peptide α-helices and optoelectronic properties. J. Am. Chem. Soc. 2011, 133, 8564–8573. [Google Scholar] [CrossRef]
- Kawamoto, H. The History of Liquid-Crystal Displays. Proc. IEEE 2002, 90, 460–500. [Google Scholar] [CrossRef]
- Nishii, M.; Matsuoka, T.; Kamikawa, Y.; Kato, T. Thermotropic liquid-crystalline peptide derivatives: Oligo(glutamic acid)s forming hydrogen-bonded columns. Org. Biomol. Chem. 2005, 3, 875–880. [Google Scholar] [CrossRef]
- Kamikawa, Y.; Kato, T. One-dimensional chiral self-assembly of pyrene derivatives based on dendritic oligopeptides. Org. Lett. 2006, 8, 2463–2466. [Google Scholar] [CrossRef]
- Gao, B.; Li, H.; Xia, D.; Sun, S.; Ba, X. Amphiphilic dendritic peptides: Synthesis and behavior as an organogelator and liquid crystal. Beilstein J. Org. Chem. 2011, 7, 198–203. [Google Scholar] [CrossRef]
- Hamley, I.W. Liquid crystal phase formation by biopolymers. Soft Matter 2010, 6, 1863–1871. [Google Scholar] [CrossRef]
- Robinson, C. Liquid-crystalline structures in polypeptide solutions. Tetrahedron 1961, 13, 219–234. [Google Scholar] [CrossRef]
- Reich, Z.; Wachtel, E.J.; Minsky, A. Liquid-crystalline mesophases of plasmid DNA in bacteria: Regulation of DNA supramolecular organization by supercoiling. Science 1994, 264, 1460–1463. [Google Scholar]
- Livolant, F. Ordered phases of DNA in vivo and in vitro. Physica A 1991, 176, 117–137. [Google Scholar] [CrossRef]
- Livolant, F.; Leforestier, A. Condensed phases of DNA: Structures and phase transitions. Prog. Polym. Sci. 1996, 21, 1115–1164. [Google Scholar] [CrossRef]
- Rey, A.D. Liquid crystal models of biological materials and processes. Soft Matter 2010, 6, 3402–3429. [Google Scholar] [CrossRef]
- Ciferri, A. A supermolecular polymerization model for the structurisation of DNA-lipid complexes. Liquid Crystals 2012, 39, 1231–1236. [Google Scholar] [CrossRef]
- Zidovska, A.; Evans, H.M.; Ewert, K.K.; Quispe, J.; Carragher, B.; Potter, C.S.; Safinya, C.R. Liquid crystalline phases of dendritic lipid-DNA self-assemblies: Lamellar, hexagonal, and DNA bundles. J. Phys. Chem. B 2009, 113, 3694–3703. [Google Scholar] [CrossRef]
- Safinya, C.R.; Ewert, K.K.; Leal, C. Cationic liposome-nucleic acid complexes: Liquid crystal phases with applications in gene therapy. Liquid Crystals 2011, 38, 1715–1723. [Google Scholar] [CrossRef]
- Powell, M.F.; Fleitman, J.; Sanders, L.M.; Si, V.C. Peptide liquid crystals: Inverse correlation of kinetic formation and thermodynamic stability in aqueous solution. Pharm. Res. 1994, 11, 1352–1354. [Google Scholar] [CrossRef]
- Engels, T.; von Rybinski, W. Liquid crystalline surfactant phases in chemical applications. J. Mater. Chem. 1998, 8, 1313–1320. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. Int. Ed. Engl. 2006, 45, 38–68. [Google Scholar] [CrossRef]
- Mizoshita, N.; Suzuki, Y.; Kishimoto, K.; Hanabusa, K.; Kato, T. Electrooptical properties of liquid-crystalline physical gels: A new oligo(amino acid) gelator for light scattering display materials. J. Mater. Chem. 2002, 12, 2197–2201. [Google Scholar] [CrossRef]
- Mizoshita, N.; Hanabusa, K.; Kato, T. Fast and high-contrast electro-optical switching of liquid-crystalline physical gels: Formation of oriented microphase-separated structures. Adv. Funct. Mater. 2003, 13, 313–317. [Google Scholar] [CrossRef]
- Mizoshita, N.; Hanabusa, K.; Kato, T. Self-Aggregation of an Amino Acid Derivative as a Route to Liquid-Crystalline Physical Gels - Faster Response to Electric Fields. Adv. Mater. 1999, 11, 392–394. [Google Scholar] [CrossRef]
- Hirai, Y.; Mizoshita, N.; Moriyama, M.; Kato, T. Self-Assembled Fibers Photopolymerized in Nematic Liquid Crystals: Stable Electrooptical Switching in Light-Scattering Mode. Langmuir 2009, 25, 8423–8427. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mizoshita, N.; Hanabusa, K.; Kato, T. Homeotropically Oriented Nematic Physical Gels for Electrooptical Materials. J. Mater. Chem. 2003, 13, 2870–2874. [Google Scholar] [CrossRef]
- Mizoshita, N.; Suzuki, Y.; Kishimoto, K.; Kato, T.; Hanabusa, K. Light Scattering Electrooptic Behavior of Liquid-Crystalline Physicail Gels - Effects of Microphase-Separated Morphologies. Mol. Cryst. Liq. Cryst. 2004, 409, 175–181. [Google Scholar] [CrossRef]
- Kato, T. Self-Assembly of Phase-Segregated Liquid Crystal Structures. Science 2002, 295, 2414–2418. [Google Scholar] [CrossRef]
- Mizoshita, N.; Suzuki, Y.; Hanabusa, K.; Kato, T. Bistable Nematic Liquid Crystals with Self-Assembled Fibers. Adv. Mater. 2005, 17, 692–696. [Google Scholar] [CrossRef]
- Nair, G.G.; Prasad, S.K.; Jayalakshmi, V.; Shanker, G.; Yelamaggad, C.V. Fast Responding Robust Nematic Liquid Crystalline Gels Formed by a Monodisperse Dipeptide: Electro-Opric and Rheological Studies. J. Phys. Chem. B 2009, 113, 6647–6651. [Google Scholar] [CrossRef]
- Bhargavi, R.; Nair, G.G.; Prasad, S.K.; Prabhu, R.; Yelamaggad, C.V. Anomalously large bend elastic constant and faster electro-optic response in anisotropic gels formed by a dipeptide. J. Appl. Phys. 2011, 109, 083537. [Google Scholar] [CrossRef]
- Sawada, N.; Iwabata, K.; Ino, K.; Sugai, U.; Seki, Y.; Kakinuma, D.; Furue, H.; Takatoh, K.; Kobayashi, S.; Sakaguchi, K. Frequency Modulation Response of a Liquid Crystal Electrooptic Device Doped with Guanine Oligonucleotides. Jpn. J. Appl. Phys. 2011, 50, 100210. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Toshima, N.; Maeda, K.; Yoshikawa, H.; Xu, J.; Kobayashi, S. Frequency modulation response of a liquid-crystal electro-optic device doped with nanoparticles. Appl. Phys. Lett. 2002, 81, 2845–2847. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Maeda, K.; Shiraishi, Y.; Xu, J.; Shiraki, H.; Toshima, N.; Kobayashi, S. Frequency Modulation Response of a Tunable Birefringent Mode Nematic Liquid Crystal Electrooptic Device Fabricated by Doping Nanoparticles of Pd Covered with Liquid-Crystal Molecules. Jpn. J. Appl. Phys. 2002, 41, L1315. [Google Scholar] [CrossRef]
- Miyama, T.; Thisayukta, J.; Shiraki, H.; Sakai, Y.; Shiraishi, Y.; Toshima, N.; Kobayashi, S. Fast Switching of Frequency Modulation Twisted Nematic Liquid Crystal Display Fabricated by Doping Nanoparticles and Its Mechanism. Jpn. J. Appl. Phys. 2004, 43, 2580–2584. [Google Scholar] [CrossRef]
- Thisayukta, J.; Shiraki, H.; Sakai, Y.; Masumi, T.; Kundu, S.; Shiraishi, Y.; Toshima, N.; Kobayashi, S. Dielectric Properties of Frequency Modulation Twisted Nematic LCDs Doped with Silver Nanoparticles. Jpn. J. Appl. Phys. 2004, 43, 5430–5434. [Google Scholar] [CrossRef]
- Shiraki, H.; Kundu, S.; Sakai, Y.; Masumi, T.; Shiraishi, Y.; Toshima, N.; Kobayashi, S. Dielectric Properties of Frequency Modulation Twisted Nematic LCDs Doped with Palladium (Pd) Nanoparticles. Jpn. J. Appl. Phsy. 2004, 43, 5425–5429. [Google Scholar] [CrossRef]
- Furue, H.; Kakinuma, D.; Toizumi, R.; Fujita, Y.; Iwabata, K.; Sakaguchi, K. Effect of DNA Doping on Liquid Crystal. Mol. Cryst. Liquid Cryst. 2011, 540, 213–218. [Google Scholar] [CrossRef]
- Eelkema, R.; Feringa, B.L. Amplification of chirality in liquid crystals. Org. Biomol. Chem. 2006, 4, 3729–3745. [Google Scholar] [CrossRef]
- Iwabata, K.; Nakabayashi, T.; Uchiyama, Y.; Inoue, M.; Taki, S.; Ando, K.; Sakai, H.; Abe, M.; Itagaki, M.; Furue, H.; Kobayashi, S.; Sakaguchi, K. Dielectric Relaxation Analysis of Single-Stranded DNA in Liquid Crystals. Jpn. J. Appl. Phsy. 2010, 49, 87002. [Google Scholar] [CrossRef]
- Kobayashi, S.; Miyama, T.; Nishida, N.; Sakai, Y.; Shiraki, H.; Shiraishi, Y.; Toshima, N. Dielectric Spectroscopy of Metal Nanoparticle Doped Liquid Crystal Displays Exhibiting Frequency Modulation Response. J. Disp. Technol. 2006, 2, 121–129. [Google Scholar] [CrossRef]
- Meggers, E.; Michel-Beyerle, M.E.; Giese, B. Sequence Dependent Long Range Hole Transport in DNA. J. Am. Chem. Soc. 1998, 120, 12950–12955. [Google Scholar] [CrossRef]
- Giese, B. Long-Distance Charge Transport in DNA: The Hopping Mechanism. Acc. Chem. Res. 2000, 33, 631–636. [Google Scholar] [CrossRef]
- Giese, B. Electron transfer in DNA. Curr. Opin. Chem. Biol. 2002, 6, 612–618. [Google Scholar] [CrossRef]
- Barnett, R.N.; Cleveland, C.L.; Landman, U.; Boone, E.; Kanvah, S.; Schuster, G.B. Effect of Base Sequence and Hydration on the Electronic and Hole Transport Properties of Duplex DNA: Theory and Experiment. J. Phys. Chem. A 2003, 107, 3525–3537. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, P.; Li, X.; Tao, N. Direct Conductance Measurement of Single DNA Molecules in Aqueous Solution. Nano Lett. 2004, 4, 1105–1108. [Google Scholar] [CrossRef]
- Xu, M.S.; Endres, R.G.; Tsukamoto, S.; Kitamura, M.; Ishida, S.; Arakawa, Y. Conformation and Local Environment Dependent Conductance of DNA Molecules. Small 2005, 1, 1168–1172. [Google Scholar] [CrossRef]
- Sugiyama, H.; Saito, I. Theoretical Studies of GG-Specific Photocleavage of DNA via Electron Transfer: Significant Lowering of Ionization Potential and 5'-Localization of HOMO of Stacked GG Bases in B-Form DNA. J. Am. Chem. Soc. 1996, 118, 7063–7068. [Google Scholar] [CrossRef]
- Tada, T.; Kondo, M.; Yoshizawa, K. You have full text access to this contentTheoretical Measurements of Conductance in an (AT)12 DNA Molecule. ChemPhysChem 2003, 4, 1256–1260. [Google Scholar] [CrossRef]
- Starikov, E.B.; Tanaka, S.; Kurita, N.; Sengoku, Y.; Natsume, T.; Wenzel, W. Investigation of a Kubo-formula-based approach to estimate DNA conductance in an atomistic model. Eur. Phys. J. E 2005, 18, 437–445. [Google Scholar] [CrossRef]
- Starikov, E.B.; Fujita, T.; Watanabe, H.; Sengoku, Y.; Tanaka, S.; Wenzel, W. Effects of molecular motion on charge transfer/transport through DNA duplexes with and without base pair mismatch. Mol. Simulation 2006, 32, 759–764. [Google Scholar] [CrossRef]
- Starikov, E.B.; Quintilla, A.; Nganou, C.; Lee, K.H.; Cuniberti, G.; Wenzel, W. Single-molecule DNA conductance in water solutions: Role of DNA low-frequency dynamics. Chem. Phys. Lett. 2009, 467, 369–374. [Google Scholar] [CrossRef]
- Lewis, J.P.; Cheatham, T.E.; Starikov, E.B.; Wang, H.; Sankey, O.F. Dynamically Amorphous Character of Electronic States in Poly(dA)-Poly(dT) DNA. J. Phys. Chem. B 2003, 107, 2581–2587. [Google Scholar] [CrossRef]
- Troisi, A.; Orlandi, G. Hole Migration in DNA: A Theoretical Analysis of the Role of Structural Fluctuations. J. Phys. Chem. B 2002, 106, 2093–2101. [Google Scholar] [CrossRef]
- Voityuk, A.A.; Siriwong, K.; Rösch, N. You have full text access to this contentEnvironmental Fluctuations Facilitate Electron-Hole Transfer from Guanine to Adenine in DNA π Stacks. Angew. Chem. Int. Ed. Engl. 2004, 43, 624–627. [Google Scholar] [CrossRef]
- Voityuk, A.A. Assessment of semiempirical methods for the computation of charge transfer in DNA π-stacks. Chem. Phys. Lett. 2006, 427, 177–180. [Google Scholar] [CrossRef]
- Van Zalinge, H.; Schiffrin, D.J.; Bates, A.D.; Starikov, E.B.; Wenzel, W.; Nichols, R.J. Variable-Temperature Measurements of the Single-Molecule Conductance of Double-Stranded DNA. Angew. Chem. Int. Ed. Engl. 2006, 45, 5499–5502. [Google Scholar] [CrossRef]
- Zalinge, H.V.; Schiffrin, D.J.; Bates, A.D.; Haiss, W.; Ulstrup, J.; Nichols, R.J. Single-Molecule Conductance Measurements of Single- and Double-Stranded DNA Oligonucleotides. ChemPhysChem 2006, 7, 94–98. [Google Scholar] [CrossRef]
- Sadowska-Aleksiejew, A.; Rak, J.; Voityuk, A.A. Effects of intra base-pairs flexibility on hole transfer coupling in DNA. Chem. Phys. Lett. 2006, 429, 546–550. [Google Scholar] [CrossRef]
- Kelley, S.O.; Boon, E.M.; Barton, J.K.; Jackson, N.M.; Hill, M.G. Single-base mismatch detection based on charge transduction through DNA. Nucleic Acids Res. 1999, 27, 4830–4837. [Google Scholar] [CrossRef]
- Okada, A.; Yokojima, S.; Kurita, N.; Sengoku, Y.; Tanaka, S. Charge transfer in duplex DNA containing mismatch. J. Mol. Struct. THEOCHEM 2003, 630, 283–290. [Google Scholar] [CrossRef]
- Asai, Y. Theory of Electric Conductance of DNA Molecule. J. Phys. Chem. B 2003, 107, 8716. [Google Scholar] [CrossRef]
- Barnett, R.N.; Cleveland, C.L.; Joy, A.; Landman, U.; Schuster, G.B. Charge Migration in DNA: Ion-Gated Transport. Science 2001, 294, 567–571. [Google Scholar] [CrossRef]
- Kubar, T.; Woiczikowski, P.B.; Cuniberti, G.; Elstner, M. Efficient Calculation of Charge-Transfer Matrix Elements for Hole Transfer in DNA. J. Phys. Chem. B 2008, 112, 7937–7947. [Google Scholar] [CrossRef]
- Kubar, T.; Elstner, M. What Governs the Charge Transfer in DNA? The Role of DNA Conformation and Environment. J. Phys. Chem. B 2008, 112, 8788–8798. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Grozema, F.C.; Guerra, C.F.; Bickelhaupt, F.M.; Lewis, F.D.; Berlin, Y.A.; Ratner, M.A.; Siebbeles, L.D.A. Absolute Rates of Hole Transfer in DNA. J. Am. Chem. Soc. 2005, 127, 14894–14903. [Google Scholar] [CrossRef]
- Roca-Sanjuan, D.; Merchan, M.; Serrano-Andres, L. Mechanistic insights into the H + O2→OH + O reaction from quasi-classical trajectory studies on a new ab initio potential energy surface. Chem. Phys. 2008, 349, 188–187. [Google Scholar] [CrossRef]
- Roche, S. Sequence Dependent DNA-Mediated Conduction. Phys. Rev. Lett. 2003, 91, 108101–108104. [Google Scholar] [CrossRef]
- Guo, A.M. Long-range correlation and charge transfer efficiency in substitutional sequences of DNA molecules. Phys. Rev. E 2007, 75, 061915–061922. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Ishikawa, Y.; Vilkas, M.J.; Natsume, T.; Dedachi, K.; Kurita, N. Density-functional theoretical study on hydrated DNA duplex: Effect of hydrating water molecules on HOMO distribution in DNA. Chem. Phys. Lett. 2006, 429, 563–569. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Ishikawa, Y.; Natsume, T.; Dedachi, K.; Kurita, N. A combined molecular dynamics/density-functional theoretical study on the structure and electronic properties of hydrating water molecules in the minor groove of decameric DNA duplex. Chem. Phys. Lett. 2007, 441, 136–142. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Wakabayashi, H.; Sengoku, Y.; Kurita, N. Charge conducting properties of hydrated DNA duplex between Au electrodes obtained by molecular simulations and Green’s function theory. Int. J. Quantum Chem. 2009, 109, 2058–2067. [Google Scholar] [CrossRef]
- Yanov, I.; Palacios, J.J.; Hill, G. Simple STM Tip Functionalization for Rapid DNA Sequencing: An Ab Initio Green’s Function Study. J. Phys. Chem. A 2008, 112, 2069–2073. [Google Scholar] [CrossRef]
- Inoue, M.; Miyake, T.; Akimoto, M.; Kobayashi, S.; Takatoh, K. Changes Caused to Asymmetric I-V Characteristics of LC Cells Having a Single-Sided Alignment Layer Structure by Doping 4-Dimethyl-aminopyridine (4-DMAP) into an LC Layer. Mol. Cryst. Liq. Cryst. 2009, 510, 312–322. [Google Scholar]
- Iwabata, K.; Seki, Y.; Toizumi, R.; Shimada, Y.; Inoue, M.; Furue, H.; Sakaguchi, K. Ion Density Analysis of Single-Stranded DNA in Liquid Crystal. Jpn. J. Appl. Phys. Submitted.
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal Materials Find a New Order in Biomedical Applications. Nat. Mater. 2007, 12, 929–938. [Google Scholar] [CrossRef]
- Lowe, A.M.; Abbott, N.L. Liquid Crystalline Materials for Biological Applications. Chem. Mater. 2012, 24, 746–758. [Google Scholar] [CrossRef]
- Brake, J.M.; Daschner, M.K.; Luk, Y.Y.; Abbott, N.L. Biomolecular Interactions at Phospholipid-Decorated Surfaces of Liquid Crystals. Science 2003, 302, 2094–2097. [Google Scholar] [CrossRef]
- Kinsinger, M.I; Lynn, D.M.; Abbott, N.L. Nematic ordering drives the phase separation of mixed monolayers containing phospholipids modified with poly(ethylene glycol) at aqueous—Liquid crystal interfaces. Soft Matter 2010, 6, 4095–4104. [Google Scholar] [CrossRef]
- Park, J.S.; Abbott, N.L. Ordering Transitions in Thermotropic Liquid Crystals Induced by the Interfacial Assembly and Enzymatic Processing of Oligopeptide Amphiphiles. Adv. Mater. 2008, 20, 1185–1190. [Google Scholar] [CrossRef]
- Park, J.S.; Teren, S.; Tepp, W.H.; Beebe, D.J.; Johnson, E.A.; Abbott, N.L. Formation of Oligopeptide-Based Polymeric Membranes at Interfaces between Aqueous Phases and Thermotropic Liquid Crystals. Chem. Mater. 2006, 18, 6147–6151. [Google Scholar] [CrossRef]
- Lundgren, J.S.; Watkins, A.N.; Racz, D.; Ligler, F.S. A liquid crystal pixel array for signal discrimination in array biosensors. Biosens. Bioelectron. 2000, 15, 417–421. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iwabata, K.; Sugai, U.; Seki, Y.; Furue, H.; Sakaguchi, K. Applications of Biomaterials to Liquid Crystals. Molecules 2013, 18, 4703-4717. https://doi.org/10.3390/molecules18044703
Iwabata K, Sugai U, Seki Y, Furue H, Sakaguchi K. Applications of Biomaterials to Liquid Crystals. Molecules. 2013; 18(4):4703-4717. https://doi.org/10.3390/molecules18044703
Chicago/Turabian StyleIwabata, Kazuki, Urara Sugai, Yasutaka Seki, Hirokazu Furue, and Kengo Sakaguchi. 2013. "Applications of Biomaterials to Liquid Crystals" Molecules 18, no. 4: 4703-4717. https://doi.org/10.3390/molecules18044703
APA StyleIwabata, K., Sugai, U., Seki, Y., Furue, H., & Sakaguchi, K. (2013). Applications of Biomaterials to Liquid Crystals. Molecules, 18(4), 4703-4717. https://doi.org/10.3390/molecules18044703