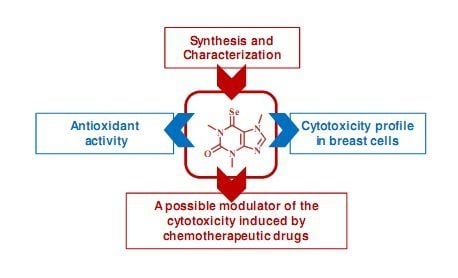
Synthesis and Biological Activity of 6-Selenocaffeine: Potential Modulator of Chemotherapeutic Drugs in Breast Cancer Cells
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis and Structural Characterization of 6-Selenocaffeine
| WR (µmol) | Solvent | Irradiation Time (min) | Max. Temp. (°C) | Irradiation potency (W) | 2 ɳ (%) |
|---|---|---|---|---|---|
| 103 | acetonitrile | 5 | 150 | 175 | 4 |
| 206 | 5 | 150 | 175 | n.r. a | |
| 103 | 5 | 130 | 200 | n.r. a | |
| 206 | 10 | 130 | 200 | n.r. a | |
| 103 | 5 | 130 | 175 | 2 | |
| 103 | 5 | 150 | 250 | 3 | |
| 103 | 10 | 150 | 250 | 5 | |
| 103 | 5 | 170 | 300 | 2 | |
| 103 | toluene | 50 | 170 | 300 | 7 |
| 103 | 90 | 170 | 300 | 17 | |
| 206 | 180 | 170 | 300 | 23 | |
| 206 | 180 | 170 | 300 | 30 | |
| 156 | p-xylene | 180 | 170 | 300 | 42 |
| 206 | 1,4-dioxane | 180 | 170 | 300 | 19 |
| 206 | propionitrile | 180 | 170 | 300 | 21 |
| Carbon | Caffeine (1) | 6-Selenocaffeine (2) | ||
|---|---|---|---|---|
| 1H-NMR a | 13C-NMR a | 1H-NMR a | 13C-NMR a | |
| δ (ppm) | δ (ppm) | δ (ppm) | δ (ppm) | |
| 2 | --- | 150.9 | --- | 149.1 |
| 4 | --- | 148.0 | --- | 143.8 |
| 5 | --- | 106.5 | --- | 121.2 |
| 6 | --- | 154.4 | --- | 175.8 |
| 8 | 7.97 | 142.7 | 8.35 | 147.4 |
| 10 | 3.12 | 27.4 | 3.75 | 37.4 |
| 11 | 3.36 | 29.3 | c.a. 3.38 | 30.3 |
| 12 | 3.84 | 33.1 | 4.10 | 35.8 |

2.2. Antioxidant Activity of 6-Selenocaffeine (2)

2.3. Assessment of the Cytotoxicity Profile of 6-Selenocaffeine (2) in Breast Cells

2.4. 6-Selenocaffeine (2) as a Possible Modulator of the Cytotoxicity Induced by Chemotherapeutic Drugs

3. Experimental
3.1. Chemicals
3.2. Instrumentation
3.3. Caffeine Selenation Mediated by WR under Microwave Irradiation
3.3.1. General Method for Optimization of Experimental Conditions
3.3.2. Optimized Conditions
3.4. Uracil Selenation Using Optimized Conditions
3.5. Caffeine Selenation under Conventional Heating
3.6. DPPH Free Radical Scavenging Assay
3.7. Cell Culture
3.8. Cytotoxicity Evaluation
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Jeong, L.S.; Tosh, D.K.; Choi, W.J.; Lee, S.K.; Kang, Y.-J.; Choi, S.; Lee, J.H.; Lee, H.; Lee, H.W.; Kim, H.O. Discovery of a new template for anticancer agents: 2'-deoxy-2'-fluoro-4'-selenoarabinofuranosyl-cytosine (2'-F-4'-Seleno-ara-C). J. Med. Chem. 2009, 52, 5303–5306. [Google Scholar] [CrossRef]
- Chou, L.-C.; Huang, L.-J.; Hsu, M.-H.; Fang, M.-C.; Yang, J.-S.; Zhuang, S.-H.; Lin, H.-Y.; Lee, F.-Y.; Teng, C.-M.; Kuo, S.-C. Synthesis of 1-benzyl-3-(5-hydroxymethyl-2-furyl)selenolo[3,2-c]pyrazole derivatives as new anticancer agents. Eur. J. Med. Chem. 2010, 45, 1395–1402. [Google Scholar] [CrossRef]
- Ibáñez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel symmetrical alkylthio- and alkylseleno-imidocarbamates. Eur. J. Med. Chem. 2011, 46, 265–274. [Google Scholar] [CrossRef]
- Chen, C.-T.; Hsu, M.-H.; Cheng, Y.-Y.; Liu, C.-Y.; Chou, L.-C.; Huang, L.-J.; Wu, T.-S.; Yang, X.; Lee, K.-H.S.; Kuo, S.-C. Synthesis and in vitro anticancer activity of 6,7-methylenedioxy (or 5-hydroxy-6-methoxy)-2-(substituted selenophenyl)quinolin-4-one analogs. Eur. J. Med. Chem. 2011, 46, 6046–6056. [Google Scholar] [CrossRef]
- Bijian, K.; Zhang, Z.; Xu, B.; Jie, S.; Chen, B.; Wan, S.; Jiang, T.; Alaoui-Jamali, M.A.; Wu, J.H. Synthesis and biological activity of novel organoselenium derivatives targeting multiple kinases and capable of inhibiting cancer progression to metastases. Eur. J. Med. Chem. 2012, 48, 143–152. [Google Scholar] [CrossRef]
- Detty, M.R.; Prasad, P.N.; Donnelly, D.J.; Ohulchanskyy, T.; Gibson, S.L.; Hilf, R. Synthesis, properties, and photodynamic properties in vitro of heavy-chalcogen analogues of tetramethylrosamine. Bioorg. Med. Chem. 2004, 12, 2537–2544. [Google Scholar] [CrossRef]
- Nian, H.; Bisson, W.H.; Dashwood, W.-M.; Pinto, J.T.; Dashwood, R.H. α-Keto acid metabolites of organoselenium compounds inhibit histone deacetylase activity in human colon cancer cells. Carcinogenesis 2009, 30, 1416–1423. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Fu, J.; Yin, H.; Xiong, K.; Tan, Q.; Li, J.; Wang, T.; Tang, W.; Yin, J.; et al. Ethaselen: A potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radical Biol. Med. 2012, 52, 898–908. [Google Scholar] [CrossRef]
- Shahabuddin, M.S.; Nambiar, M.; Choudhary, B.; Advirao, G.M.; Raghavan, S.C. A novel DNA intercalator, butylamino-pyrimido[4',5':4,5]selenolo(2,3-b)quinoline, induces cell cycle arrest and apoptosis in leukemic cells. Invest. New Drugs 2010, 28, 35–48. [Google Scholar] [CrossRef]
- Ninomiya, M.; Garud, D.R.; Koketsu, M. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011, 255, 2968–2990. [Google Scholar] [CrossRef]
- Johansson, H.; Svartström, O.; Phadnis, P.; Engman, L.; Ott, M.K. Exploring a synthetic organoselenium compound for antioxidant pharmacotherapy—Toxicity and effects on ROS-production. Bioorg. Med. Chem. 2010, 18, 1783–1788. [Google Scholar] [CrossRef]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R. Mechanisms of mammary cancer chemoprevention by organoselenium compounds. Mutat. Res. 2004, 551, 181–197. [Google Scholar] [CrossRef]
- Naithani, R. Organoselenium compounds in cancer chemoprevention. Mini-Rev. Med. Chem. 2008, 8, 657–668. [Google Scholar] [CrossRef]
- Sanmartin, C.; Plano, D.; Palop, J.A. Selenium compounds and apoptotic modulation: A new perspective in cancer therapy. Mini-Rev. Med. Chem. 2008, 8, 1020–1031. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A. Is caffeine a good scavenger of oxygenated free radicals? J. Phys. Chem. B 2011, 115, 4538–4546. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007, 247, 26–39. [Google Scholar] [CrossRef]
- Murai, T.; Kato, S. Selenocarbonyls. In Topics in Current Chemistry; Wirth, T., Ed.; Springer-Verlag: Berlin, Germany, 2000; Volume 208, pp. 177–199. [Google Scholar]
- Bhattacharyya, P.; Woollins, J.D. Selenocarbonyl synthesis using Woollins reagent. Tet. Lett. 2001, 42, 5949–5951. [Google Scholar] [CrossRef]
- López-García, M.Á. Woollins’ Reagent. Synlett 2009, 2373–2374. [Google Scholar]
- Bethke, J.; Karaghiosoff, K.; Wessjohann, L.A. Synthesis of N,N-disubstituted selenoamides by O/Se-exchange with selenium–Lawesson’s reagent. Tet. Lett. 2003, 44, 6911–6913. [Google Scholar] [CrossRef]
- Varma, R.S.; Kumar, D. Microwave-accelerated solvent-free synthesis of thioketones, thiolactones, thioamides, thionoesters, and thioflavonoids. Org. Lett. 1999, 1, 697–700. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Zhang, W.W.; Nguyen, K.P.P.; Kamounah, F.S.; Hansen, P.E. NMR of a series of novel hydroxyflavothiones. Magn. Reson. Chem. 2009, 47, 1043–1054. [Google Scholar] [CrossRef]
- Duddeck, H. Selenium-77 nuclear magnetic resonance spectroscopy. Prog. Nucl. Mag. Res. Spectrosc. 1995, 27, 1–323. [Google Scholar] [CrossRef]
- Schneider, M.; Gil, M.J.; Reliquet, A.; Meslin, J.C.; Levillain, J.; Vazeux, M.; Jury, D.; Mieloszynski, J.L.; Paquer, D. Correlations des déplacements chimiques en RMN 13C de composés carbonyles, thiocarbonyles et selenocarbonyles. Phosphorus Sulfur 1998, 134/135, 295–305. [Google Scholar]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, BR325–BR330. [Google Scholar]
- Brezová, V.; Šlebodová, A.; Staško, A. Coffee as a source of antioxidants: An EPR study. Food Chem. 2009, 114, 859–868. [Google Scholar] [CrossRef]
- Rivelli, D.P.; Silva, V.V.; Ropke, C.D.; Miranda, D.V.; Almeida, R.L.; Sawada, T.C.H.; Barros, S.B.M. Simultaneous determination of chlorogenic acid, caffeic acid and caffeine in hydroalcoholic and aqueous extracts of Ilex paraguariensis by HPLC and correlation with antioxidant capacity of the extracts by DPPH· reduction. Braz. J. Pharm. Sci. 2007, 43, 215–222. [Google Scholar]
- Anesini, C.; Turner, S.; Cogoi, L.; Filip, R. Study of the participation of caffeine and polyphenols on the overall antioxidant activity of mate (Ilex paraguariensis). LWT-Food Sci. Technol. 2012, 45, 299–304. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Rocha, J.B. Toxicology and pharmacology of selenium: Emphasis on synthetic organoselenium compounds. Arch. Toxicol. 2011, 85, 1313–1359. [Google Scholar] [CrossRef]
- Merino-Montiel, P.; Maza, S.; Martos, S.; López, Ó.; Maya, I.; Fernández-Bolaños, J.S. Synthesis and antioxidant activity of O-alkyl selenocarbamates, selenoureas and selenohydantoins. Eur. J. Pharm. Sci. 2013, 48, 582–592. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Gaspar, J.; Cabral, M.F.; Caneiras, C.; Guedes, R.; Rueff, J.; Castro, M.; Costa, J.; Oliveira, N.G. Macrocyclic copper (II) complexes: Superoxide scavenging activity, structural studies and cytotoxicity evaluation. J. Inorg. Biochem. 2007, 101, 849–858. [Google Scholar] [CrossRef]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: a double-edged sword for defense and offence in cancer. Arch Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef]
- Ramos, D.L.; Gaspar, J.F.; Pingarilho, M.; Gil, O.M.; Fernandes, A.S.; Rueff, J.; Oliveira, N.G. Genotoxic effects of doxorubicin in cultured human lymphocytes with different glutathione S-transferase genotypes. Mutat. Res. 2011, 724, 28–34. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Serejo, J.; Gaspar, J.; Cabral, F.; Bettencourt, A.F.; Rueff, J.; Castro, M.; Costa, J.; Oliveira, N.G. Oxidative injury in V79 Chinese hamster cells: protective role of the superoxide dismutase mimetic MnTM-4-PyP. Cell Biol. Toxicol. 2010, 26, 91–101. [Google Scholar] [CrossRef]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Costa, J.; Gaspar, J.; Rueff, J.; Cabral, M.F.; Cipriano, M.; Castro, M.; Oliveira, N.G. Development of pyridine-containing macrocyclic copper(II) complexes: Potential role in the redox modulation of oxaliplatin toxicity in human breast cells. Free Radical Res. 2012, 46, 1157–1166. [Google Scholar] [CrossRef]
- Hill, G.M.; Moriarity, D.M.; Setzer, W.N. Attenuation of cytotoxic natural product DNA intercalating agents by caffeine. Sci. Pharm. 2011, 79, 729–747. [Google Scholar] [CrossRef]
- Wood, P.T.; Woollins, J.D. Phosphorus–selenium heterocycles. J. Chem. Soc. Chem. Commun. 1988, 1190–1191. [Google Scholar] [CrossRef]
- Shiue, C.-Y.; ChuA, S.-H. Facile Synthesis of l-β-D-Arabinofuranosyl-2-seleno- and -4-selenouracil and related compounds. J. Org. Chem. 1975, 40, 2971–2974. [Google Scholar] [CrossRef]
- Acker, C.I.; Brandão, R.; Rosário, A.R.; Nogueira, C.W. Antioxidant effect of alkynylselenoalcohol compounds on liver and brain of rats in vitro. Environ. Toxicol. Pharm. 2009, 28, 280–287. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 6-selenocaffeine (2) and 6-selenouracil are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martins, I.L.; Miranda, J.P.; Oliveira, N.G.; Fernandes, A.S.; Gonçalves, S.; Antunes, A.M.M. Synthesis and Biological Activity of 6-Selenocaffeine: Potential Modulator of Chemotherapeutic Drugs in Breast Cancer Cells. Molecules 2013, 18, 5251-5264. https://doi.org/10.3390/molecules18055251
Martins IL, Miranda JP, Oliveira NG, Fernandes AS, Gonçalves S, Antunes AMM. Synthesis and Biological Activity of 6-Selenocaffeine: Potential Modulator of Chemotherapeutic Drugs in Breast Cancer Cells. Molecules. 2013; 18(5):5251-5264. https://doi.org/10.3390/molecules18055251
Chicago/Turabian StyleMartins, Inês L., Joana P. Miranda, Nuno G. Oliveira, Ana S. Fernandes, Sandrina Gonçalves, and Alexandra M. M. Antunes. 2013. "Synthesis and Biological Activity of 6-Selenocaffeine: Potential Modulator of Chemotherapeutic Drugs in Breast Cancer Cells" Molecules 18, no. 5: 5251-5264. https://doi.org/10.3390/molecules18055251
APA StyleMartins, I. L., Miranda, J. P., Oliveira, N. G., Fernandes, A. S., Gonçalves, S., & Antunes, A. M. M. (2013). Synthesis and Biological Activity of 6-Selenocaffeine: Potential Modulator of Chemotherapeutic Drugs in Breast Cancer Cells. Molecules, 18(5), 5251-5264. https://doi.org/10.3390/molecules18055251