Analysis of Biotinylated Generation 4 Poly(amidoamine) (PAMAM) Dendrimer Distribution in the Rat Brain and Toxicity in a Cellular Model of the Blood-Brain Barrier
Abstract
:1. Introduction
2. Results and Discussion
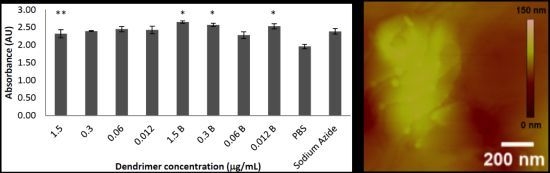
2.1. Toxicity in Co-Culture

2.2. Fluorescence Intensity of Rat Brain, Kidney, and Liver Tissues

2.3. AFM Analysis



3. Experimental
3.1. Dendrimer Preparation
3.2. Cell Co-Culture
3.3. Lactate Dehydrogenase (LDH) Toxicity Assay
3.4. Animals
3.5. Tail Vein Injections
3.6. Brain Tissue Preparation
3.7. Fluorescence Intensity in Rat Brain, Kidney and Liver Tissues
3.8. AFM Analysis of Brain Tissue
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhu, S.; Qian, L.; Pei, Y.; Qiu, Y.; Jiang, Y. Rgd-modified PEG-PAMAM-DOX conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharm. Biopharm. 2011, 79, 232–240. [Google Scholar] [CrossRef]
- Montero, A.J.; Adams, B.; Diaz-Montero, C.M.; Gluck, S. Nab-paclitaxel in the treatment of metastatic breast cancer: A comprehensive review. Expert Rev. Clin. Pharmacol. 2011, 4, 329–334. [Google Scholar] [CrossRef]
- Lim, Z.Z.; Li, J.E.; Ng, C.T.; Yung, L.Y.; Bay, B.H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef]
- Deeken, J.F.; Löscher, W. The blood-brain barrier and cancer: Transporters, treatment, and trojan horses. Clin. Cancer. Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef]
- Koziara, J.M.; Whisman, T.R.; Tseng, M.T.; Mumper, R.J. In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J. Control. Release 2006, 112, 312–319. [Google Scholar] [CrossRef]
- Yang, C.-S.; Chang, C.-H.; Tsai, P.-J.; Chen, W.-Y.; Tseng, F.-G.; Lo, L.-W. Nanoparticle-based in vivo investigation on blood−brain barrier permeability following ischemia and reperfusion. Anal. Chem. 2004, 76, 4465–4471. [Google Scholar] [CrossRef]
- Dermietzel, R.; Krause, D. Molecular anatomy of the blood-brain barrier as defined by immunocytochemistry. Int. Rev. Cytol. 1991, 127, 57–109. [Google Scholar] [CrossRef]
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- Savettieri, G.; Di Liegro, I.; Catania, C.; Licata, L.; Pitarresi, G.L.; D'Agostino, S.; Schiera, G.; de Caro, V.; Giandalia, G.; Giannola, L.I.; et al. Neurons and ecm regulate occludin localization in brain endothelial cells. Neuroreport 2000, 11, 1081–1084. [Google Scholar] [CrossRef]
- Yang, H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm. Res. 2010, 27, 1759–1771. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010, 7, 1–25. [Google Scholar] [CrossRef]
- Agarwal, A.; Lariya, N.; Saraogi, G.; Dubey, N.; Agrawal, H.; Agrawal, G.P. Nanoparticles as novel carrier for brain delivery: A review. Curr. Pharm. Des. 2009, 15, 917–925. [Google Scholar]
- Gilmore, J.L.; Xiang, Y.; Lingdong, Q.; Kabanov, A. Novel nanomaterials for clinical neuroscience. J. Neuroimmune Pharmacol. 2008, 3, 83–94. [Google Scholar] [CrossRef]
- Dykes, G.M. Dendrimers: A review of their appeal and applications. J. Chem. Technol. Biotechnol. 2001, 76, 903–918. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic organic chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Tomalia, D.A. The emergence of a new macromolecular architecture: The dendritic state. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 671–692. [Google Scholar]
- Tomalia, D.A.; Henderson, S.A.; Diallo, M.S. Dendrimers - an enabling synthetic science to controlled organic nanostructures. In Handbook of Nanoscience, Engineering and Technology; Goddard, W.A.I., Brenner, D.W., Lyshevski, S.E., Irafrate, G.J., Eds.; CRC Press: Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 24.21–24.47. [Google Scholar]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar]
- Gajbhiye, V.; Palanirajan, V.K.; Tekade, R.K.; Jain, N.K. Dendrimers as therapeutic agents: A systematic review. J. Pharm. Pharmacol. 2010, 61, 989–1003. [Google Scholar]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar]
- Wijagkanalan, W.; Kawakami, S.; Hashida, M. Designing dendrimers for drug delivery and imaging: Pharmacokinetic considerations. Pharm. Res. 2011, 28, 1500–1519. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar]
- Petkar, K.C.; Chavhan, S.S.; Agatonovik-Kustrin, S.; Sawant, K.K. Nanostructured materials in drug and gene delivery: A review of the state of the art. Crit. Reve. Therm. Drug Carrier Syst. 2011, 28, 101–164. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Han, L.; Liu, Y.; Shao, K.; Ye, L.; Lou, J.; Jiang, C.; Pei, Y. Brain-targeting mechanisms of lactoferrin-modified DNA-loaded nanoparticles. J. Cereb Blood Flow Metab 2009, 29, 1914–1923. [Google Scholar]
- Wu, G.; Barth, R.F.; Yang, W.; Kawabata, S.; Zhang, L.; Green-Church, K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225 dendrimer bioconjugates. Mol. Cancer Ther. 2006, 5, 52–59. [Google Scholar]
- Kaneshiro, T.L.; Lu, Z.R. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials 2009, 30, 5660–5666. [Google Scholar] [CrossRef]
- Spector, R.; Mock, D. Biotin transport through the blood-brain barrier. J. Neurochem. 1987, 48, 400–404. [Google Scholar] [CrossRef]
- Shi, F.; Bailey, C.; Malick, A.W.; Audus, K.L. Biotin uptake and transport across bovine brain microvessel endothelial cell monolayers. Pharm. Res. 1993, 10, 282–288. [Google Scholar]
- Beg, S.; Samad, A.; Alam, M.I.; Nazish, I. Dendrimers as novel systems for delivery of neuropharmaceuticals to the brain. CNS Neurol. Disord. Drug Targets 2011, 10, 576–588. [Google Scholar] [CrossRef]
- Bullen, H.A.; Hemmer, R.; Haskamp, A.; Cason, C.A.; Wall, S.; Spaulding, R.; Rossow, B.; Hester, M.; Caroway, M.; Haik, K.L. Evaluation of biotinylated pamam dendrimer toxicity in models of the blood brain barrier: A biophysical and cellular approach. J. Biomater. Nanobiotechnol. 2011, 2, 485–493. [Google Scholar] [CrossRef]
- Sato, N.; Kobayashi, H.; Saga, T.; Nakamoto, Y.; Ishimori, T.; Togashi, K.; Fujibayashi, Y.; Konishi, J.; Brechbiel, M.W. Tumor targeting and imaging of interaperitoneal tumors by use of antisense oligo-DNA complexed with dendrimers and/or avidin in mice. Clin. Cancer. Res. 2001, 7, 3606–3612. [Google Scholar]
- Wilbur, D.S.; Pathare, P.M.; Hamlin, D.K.; Buhler, K.R.; Vessella, R.L. Biotin reagents for antibody pretargeting. 3. Synthesis, radioiodination, and evaluation of biotinylated starburst dendrimers. Bioconjug. Chem. 1998, 9, 813–825. [Google Scholar] [CrossRef]
- Yoon, H.C.; Hong, M.Y.; Kim, H.S. Affinity biosensor for avidin using a double functionalized dendrimer monolayer on a gold electrode. Anal. Biochem. 2000, 282, 121–128. [Google Scholar] [CrossRef]
- Pericet-Camara, R.; Papastavrou, G.; Borkovec, M. Atomic force microscopy study of the adsorption and electrostatic self-organization of poly(amidoamine) dendrimers on mica. Langmuir 2004, 20, 3264–3270. [Google Scholar] [CrossRef]
- Zhang, H.; Grim, P.C.M.; Foubert, P.; Vosch, T.; Vanoppen, P.; Wiesler, U.M.; Berresheim, A.J.; Müllen, K.; de Schryver, F.C. Properties of single dendrimer molecules studied by atomic force microscopy. Langmuir 2000, 16, 9009–9014. [Google Scholar] [CrossRef]
- Melling, M.; Hochmeister, S.; Blumer, R.; Schilcher, K.; Mostler, S.; Behnam, M.; Wilde, J.; Karimian-Teherani, D. Atomic force microscopy imaging of the human trigeminal ganglion. Neuroimage 2001, 14, 1348–1352. [Google Scholar] [CrossRef]
- Nie, H.-Y.; Taylor, A.R.; Lau, W.M.; MacFabe, D.F. Subcellular features revealed on unfixed rat brain sections by phase imaging. Analyst 2011, 136, 2270–2276. [Google Scholar] [CrossRef]
- Li, J.; Piehler, L.T.; Qin, D.; Baker, J.R.; Tomalia, D.A.; Meier, D.J. Visualization and characterization of poly(amidoamine) dendrimers by atomic force microscopy. Langmuir 2000, 16, 5613–5616. [Google Scholar] [CrossRef]
- Thalhammer, A.; Edgington, R.J.; Cingolani, L.A.; Schoepfer, R.; Jackman, R.B. The use of nanodiamond monolayer coatings to promote the formation of functional neuronal networks. Biomaterials 2010, 31, 2097–2014. [Google Scholar] [CrossRef]
- Serem, W.K.; Bett, C.K.; Ngujiri, J.N.; Garno, J.C. Studies of the growth, evolution, and self-aggregation of β-amyloid fibrils using tapping-mode atomic force microscopy. Microsc. Res. Tech. 2011, 74, 699–708. [Google Scholar] [CrossRef]
- Harper, J.D.; Lieber, C.M.; Lansbury Jr, P.T. Atomic force microscopic imaging of seeded fibril formation and fibril branching by the alzheimer's disease amyloid-β protein. Chem. Biol. 1997, 4, 951–959. [Google Scholar] [CrossRef]
- Kowalewski, T.; Holtzman, D.M. In situ atomic force microscopy study of alzheimer’s β-amyloid peptide on different substrates: New insights into mechanism of β-sheet formation. Proc. Natl. Acad. Sci. USA 1999, 96, 3688–3693. [Google Scholar] [CrossRef]
- Jiang, W.; Duysen, E.G.; Hansen, H.; Shlyakhtenko, L.; Schopfe, L.M.; Lockridge, O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol. Sci. 2010, 115, 183–193. [Google Scholar] [CrossRef]
- Christ, A.F.; Franze, K.; Gautier, H.; Moshayedi, P.; Fawcett, J.; Franklin, R.J.; Karadottir, R.T.; Guck, J. Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. J. Biomech. 2010, 43, 2986–2992. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Rozanek, M.; Szewczyk, M.; Klajnert, B.; Bryszewska, M. The influence of PAMAM-OH dendrimers on the activity of human erythrocytes atpases. Biochim. Biophys. Acta 2011, 1808, 2714–2723. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.B.; D'Emanuele, A. The influenceof surface modification on the cytotoxicity of pamam dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, W.; Zhu, Y.; Xu, H.; Yang, X. Protective effect of pegylation against poly(amidoamine) dendrimer-induced hemolysis of human red blood cells. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 59–64. [Google Scholar]
- Spector, R.; Mock, D.M. Biotin transport and metabolism in the central nervous system. Neurochem. Res. 1988, 13, 213–219. [Google Scholar] [CrossRef]
- De Boer, A.G.; Gaillard, P.J. In vitro models of the blood-brain barrier: When to use which? Curr. Med. Chem. Cent. Nerv. Syst. Agents 2002, 2, 203–209. [Google Scholar] [CrossRef]
- Garberg, P.; Ball, M.; Borg, N.; Cecchelli, R.; Fenart, L.; Hurst, R.D.; Lindmark, T.; Mabondzo, A.; Nilsson, J.E.; Raub, T.J.; et al. In vitro models for the blood-brain barrier. Toxicol. In Vitro 2005, 19, 299–334. [Google Scholar] [CrossRef]
- Avalos Funez, A.; Isabel Haza, A.; Mateo, D.; Morales, P. In vitro evaluation of silver nanoparticles on human tumoral and normal cells. Toxicol. Mech. Methods 2013, 23, 153–160. [Google Scholar] [CrossRef]
- Weiss, V.M.; Naolou, T.; Groth, T.; Kressler, J.; Mader, K. In vitro toxicity of stearoyl-poly(glycerol adipate) nanoparticles. J. Appl. Biomater. Funct. Mater. 2012, 10, 163–169. [Google Scholar]
- Gelperina, S.; Maksimenko, O.; Khalansky, A.; Vanchugova, L.; Shipulo, E.; Abbasova, K.; Berdiev, R.; Wohlfart, S.; Chepurnova, N.; Kreuter, J. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: Influence of the formulation parameters. Eur. J. Pharm. Biopharm. 2010, 74, 157–163. [Google Scholar] [CrossRef]
- Weiss, C.; Kohnle, M.; Landfester, K.; Hauk, T.; Fischer, D.; Schmitz-Wienke, J.; Mailander, V. The first step into the brain: Uptake of NiO-PBCA nanoparticles by endothelial cells in vitro and in vivo, and direct evidence for their blood-brain barrier permeation. ChemMedChem 2008, 3, 1395–1403. [Google Scholar] [CrossRef]
- Wang, D.; Imae, T. Fluorescence emission from dendrimers and its pH dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef]
- Perumal, O.P.; Inapagolla, R.; Kannan, S.; Kannan, R.M. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials 2008, 29, 3469–3476. [Google Scholar] [CrossRef]
- Duran-Vilaregut, J.; del Valle, J.; Camins, A.; Pallas, M.; Pelegri, C.; Vilaplana, J. Blood-brain barrier disruption in the striatum of rats treated with 3-nitropropionic acid. Neurotoxicology 2009, 30, 136–143. [Google Scholar] [CrossRef]
- Duran-Vilaregut, J.; Manich, G.; del Valle, J.; Pallas, M.; Camins, A.; Pelegri, C.; Vilaplana, J. Neuronal apoptosis in the striatum of rats treated with 3-nitropropionic acid is not triggered by cell-cycle re-entry. Neurotoxicology 2011, 32, 734–741. [Google Scholar] [CrossRef]
- Prieto, M.J.; Schilrreff, P.; Tesoriero, M.V.; Morilla, M.J.; Romero, E.L. Brain and muscle of Wistarrats are the main targets of intravenous dendrimeric sulfadiazine. Int. J. Pharm. 2008, 360, 204–212. [Google Scholar] [CrossRef]
- Dai, H.; Navath, R.S.; Balakrishnan, B.; Guru, B.R.; Mishra, M.K.; Romero, R.; Kannan, R.M.; Kannan, S. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine (Lond) 2010, 5, 1317–1329. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Xu, X.; Zhang, X.; Zhu, H.; Huang, L.; Qi, Y.; Shen, Y.M. Synthesis, biodistribution, and microsingle photon emission computed tomography (spect) imaging study of technetium-99m labeled pegylated dendrimer poly(amidoamine) (PAMAM)-folic acid conjugates. J. Med. Chem. 2010, 53, 3262–3272. [Google Scholar] [CrossRef]
- Janaszewska, A.; Ziemba, B.; Ciepluch, K.; Appelhans, D.; Voit, B.; Klajnert, B.; Bryszewska, M. The biodistribution of maltotriose modified poly(propylene imine) (PPI) dendrimers conjugated with fluorescein-proofs of crossing blood-brain-barrier. New J. Chem. 2012, 36, 350–353. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Coursin, D.B. Depletion of biotin from brain and liver in biotin deficiency. J. Neurochem. 1970, 17, 289–290. [Google Scholar] [CrossRef]
- Wang, H.; Pevsner, J. Detection of endogenous biotin in various tissues: Novel functions in the hippocampus and implications for its use in avidin-biotin technology. Cell Tissue Res. 1999, 296, 511–516. [Google Scholar] [CrossRef]
- Wood, G.S.; Warnke, R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J. Histochem. Cytochem. 1981, 29, 1196–1204. [Google Scholar] [CrossRef]
- McKay, B.E.; Molineux, M.L.; Turner, R.W. Endogenous biotin in rat brain: Implications for false-positive results with avidin-biotin and streptavidin-biotin techniques. Methods Mol. Biol. 2008, 418, 111–128. [Google Scholar]
- McKay, B.E.; Molineux, M.L.; Turner, R.W. Biotin is endogenously expressed in select regions of the rat central nervous system. J. Comp. Neurol. 2004, 473, 86–96. [Google Scholar] [CrossRef]
- Cason, C.A.; Fabre, T.A.; Buhrlage, A.; Haik, K.L.; Bullen, H.A. Low-level detection of poly(amidoamine) PAMAM dendrimers using immunoimaging scanning probe microscopy. Int. J. Anal. Chem. 2012, 2012, 341260. [Google Scholar]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Hansma, H.G.; Hoh, J.H. Biomolecular imaging with the atomic force microscope. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 115–139. [Google Scholar] [CrossRef]
- Cason, C.A.; Oehrle, S.A.; Fabre, T.A.; Girten, C.; Walters, K.A.; Tomalia, D.A.; Haik, K.L.; Bullen, H.A. Improved methodology for monitoring poly(amidoamine) dendrimers surface transformations and product quality by ultra performance liquid chromatography. J. Nanomater. 2008, 2008, 456082. [Google Scholar]
- Garcia-Garcia, E.; Gil, S.; Andrieux, K.; Desmaele, D.; Nicolas, V.; Taran, F.; Georgin, D.; Andreux, J.P.; Roux, F.; Couvreur, P. A relevant in vitro rat model for the evaluation of blood-brain barrier translocation of nanoparticles. Cell Mol. Life Sci. 2005, 62, 1400–1408. [Google Scholar] [CrossRef]
- Szabo, C.A.; Deli, M.A.; Ngo, T.K.; Joo, F. Production of pure primary rat cerebral endothelial cell culture: A comparison of different methods. Neurobiology (Bp.) 1997, 5, 1–16. [Google Scholar]
- Bonfoco, E.; Krainc, D.; Ankarcrona, M.; Nicotera, P.; Lipton, S.A. Apoptosis and necrosis: Two distinct events induced, respectively, by mild and intense insults with n-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. USA 1995, 92, 7162–7166. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.-L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1998, 15, 61–69. [Google Scholar]
- Legrand, C.; Bour, J.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D. Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium. J. Biotechnol. 1995, 25, 231–243. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Sterotaxic Coordinates; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Sample Availability: Samples of the compounds are available from Dendritic Nanotechnologies, Inc. Mt. Pleasant, MI, USA.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hemmer, R.; Hall, A.; Spaulding, R.; Rossow, B.; Hester, M.; Caroway, M.; Haskamp, A.; Wall, S.; Bullen, H.A.; Morris, C.; et al. Analysis of Biotinylated Generation 4 Poly(amidoamine) (PAMAM) Dendrimer Distribution in the Rat Brain and Toxicity in a Cellular Model of the Blood-Brain Barrier. Molecules 2013, 18, 11537-11552. https://doi.org/10.3390/molecules180911537
Hemmer R, Hall A, Spaulding R, Rossow B, Hester M, Caroway M, Haskamp A, Wall S, Bullen HA, Morris C, et al. Analysis of Biotinylated Generation 4 Poly(amidoamine) (PAMAM) Dendrimer Distribution in the Rat Brain and Toxicity in a Cellular Model of the Blood-Brain Barrier. Molecules. 2013; 18(9):11537-11552. https://doi.org/10.3390/molecules180911537
Chicago/Turabian StyleHemmer, Ruth, Andrew Hall, Robert Spaulding, Brett Rossow, Michael Hester, Megan Caroway, Anthony Haskamp, Steven Wall, Heather A. Bullen, Celeste Morris, and et al. 2013. "Analysis of Biotinylated Generation 4 Poly(amidoamine) (PAMAM) Dendrimer Distribution in the Rat Brain and Toxicity in a Cellular Model of the Blood-Brain Barrier" Molecules 18, no. 9: 11537-11552. https://doi.org/10.3390/molecules180911537
APA StyleHemmer, R., Hall, A., Spaulding, R., Rossow, B., Hester, M., Caroway, M., Haskamp, A., Wall, S., Bullen, H. A., Morris, C., & Haik, K. L. (2013). Analysis of Biotinylated Generation 4 Poly(amidoamine) (PAMAM) Dendrimer Distribution in the Rat Brain and Toxicity in a Cellular Model of the Blood-Brain Barrier. Molecules, 18(9), 11537-11552. https://doi.org/10.3390/molecules180911537