Amination of Nitroazoles — A Comparative Study of Structural and Energetic Properties
Abstract
:1. Introduction

2. Results and Discussion
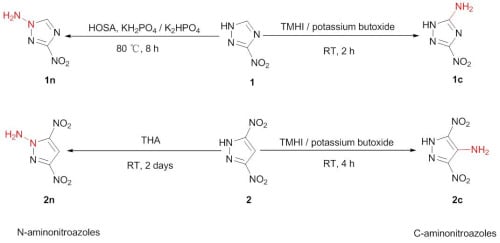
2.1. Synthesis

2.2. Spectroscopy

2.3. Crystal Structure


2.4. Physicochemical Properties
| Comp. | N a | Ω b | IS c | Td d | D e | HOF f | P g | D h |
|---|---|---|---|---|---|---|---|---|
| 1 | 49.12 | −42.11 | >40 | 218 | 1.727 | 216.9 | 28.9 | 8255 |
| 1n | 54.26 | −43.41 | >40 | 137 | 1.698 | 217.9 | 27.4 | 8194 |
| 1c | 54.26 | −43.41 | >40 | 246(241 j) | 1.819 | 201.8 | 31.5 | 8582 |
| 2 | 35.44 | −30.88 | >40 | 295 | 1.844 | 128.5 | 32.7 | 8388 |
| 2n | 40.46 | −32.37 | >40 | 112 | 1.810 | 144.4 | 31.7 | 8384 |
| 2c | 40.46 | −32.37 | >40 | 178(177 j) | 1.900 | 96.3 | 34.2 | 8573 |
| 2cn | 44.68 | −34.04 | >40 | 241 | 1.880 | 166.0 | 34.6 (35.0i) | 8712 (8732 i) |
| 3 | 60.87 | −7.00 | >1 | 130 | 1.899 | 281.0 | 39.2 (39.0i) | 9156 (9457i) |
| 3n | 64.61 | −12.30 | >1 | 140 | 1.791 | 376.4 | 36.7(36.8 i) | 9056 (9087 i) |
| TNT | 18.50 | −74.01 | 15 | 295 | 1.650 | −67.0 | 19.5 | 6881 |
| TATB | 32.55 | −55.81 | 50 | 360 | 1.930 | −154.2 | 31.2 | 8114 |
3. Experimental
3.1. General Information
3.2. 5-Amino-3-nitro-1H-1,2,4-triazole (1c)
3.3. 3,5-Dinitro-1H-pyrazole 2
3.4. 4-Amino-3,5-dinitro-1H-pyrazole 2c
3.5. 1-Amino-3-nitro-1,2,4-triazole (1n)
3.6. 1-Amino-3,5-dinitropyrazole 2n
4. Conclusions
| Group | N | Ω | D | HOF | P | D |
|---|---|---|---|---|---|---|
| N-amino | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ |
| C-amino | ↑ | ↓ | ↑ | ↓ | ↑ | ↑ |
Supplementary Materials
Acknowledgments
Conflicts of Interest
References and Notes
- Agrawal, J.P. Recent trends in high-energy materials. Prog.Energy Combust. Sci. 1998, 24, 1–30. [Google Scholar] [CrossRef]
- Huynh, M.H.V.; Hiskey, M.A.; Chavez, D.E.; Naud, D.L.; Gilardi, R.D. Synthesis, characterization, and energetic properties of diazido heteroaromatic high-nitrogen C-N compound. J. Am. Chem. Soc. 2005, 127, 12537–12543. [Google Scholar] [CrossRef]
- Brand, H.; Mayer, P.; Schulz, A.; Weigand, J.J. Nitro(nitroso)cyanomethanides. Angew. Chem.Int. Ed. 2005, 44, 3929–3932. [Google Scholar] [CrossRef]
- Tang, Y.X.; Yang, H.W.; Wu, B.; Ju, X.H.; Lu, C.X.; Cheng, G.B. Synthesis and characterization of a stable, catenated N11 energetic salt. Angew. Chem. Int. Ed. 2013, 52, 4875–4877. [Google Scholar] [CrossRef]
- Chavez, D.E.; Hiskey, M.A.; Gilardi, R.D. 3,3'-Azobis(6-amino-1,2,4,5-tetrazine): A novel high-nitrogen energetic material. Angew. Chem. 2000, 112, 1861–1863. [Google Scholar] [CrossRef]
- Huynh, M.H.V.; Hiskey, M.A.; Hartline, E.L.; Montoya, D.P.; Gilardi, R. Polyazido high-nitrogen compounds: Hydrazo- and azo-1,3,5-triazine. Angew. Chem. Int. Ed. 2004, 43, 4924–4928. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabate, C.M. Bistetrazoles: Nitrogen-rich, high-performing, insensitive energetic compounds. Chem. Mater. 2008, 20, 3629–3637. [Google Scholar] [CrossRef]
- Gao, H.X.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Gálvez-Ruiz, J.C.; Holl, G.; Karaghiosoff, K.; Klapötke, T.M.; Löhnwitz, K.; Mayer, P.; Nöth, H.; Polborn, K.; Rohbogner, C.J.; Suter, M.; et al. Derivatives of 1,5-diamino-1H-tetrazole: A new family of energetic heterocyclic based salts. Inorg. Chem. 2005, 44, 4237–4253. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. The 1,3-diamino-1,2,3-triazolium cation: A highly energetic moiety. Eur. J. Inorg. Chem. 2013, 1509–1517. [Google Scholar]
- Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Synthesis of 5-aminotetrazole-1 N-oxide and its azo derivative: A key step in the development of new energetic materials. Chem. Eur. J. 2013, 19, 4602–4613. [Google Scholar] [CrossRef]
- Herve, G.; Roussel, C.; Graindorge, H. Selective preparation of 3,4,5-trinitro-1H-pyrazole: A stable all-carbon-nitrated arene. Angew. Chem. 2010, 122, 3245–3249. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapötke, T.M. A study of dinitro-bis-1,2,4-triazole-1,1'-diol and derivatives: Design of high-performance insensitive energetic materials by the introduction of N-oxides. J. Am. Chem. Soc. 2013, 135, 9931–9938. [Google Scholar] [CrossRef]
- Göbel, M.; Karaghiosoff, K.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Nitrotetrazolate-2N-oxides and the strategy of N-oxide introduction. J. Am. Chem. Soc. 2010, 132, 17216–17226. [Google Scholar]
- Thottempudi, V.; Forohor, F.; Parrish, D.A.; Shreeve, J.M. Tris(triazolo)benzene and its derivatives: High-density energetic materials. Angew. Chem. Int. Ed. 2012, 51, 9881–9885. [Google Scholar] [CrossRef]
- Thottempudi, V.; Gao, H.; Shreeve, J.M. Trinitromethyl-substituted 5-nitro- or 3-azo-1,2,4-triazoles: Synthesis, characterization, and energetic properties. J. Am.Chem. Soc. 2011, 133, 6464–6471. [Google Scholar] [CrossRef]
- Zhang, Y.; Parrish, D.A.; Shreeve, J.M. 4-Nitramino-3,5-dinitropyrazole-based energetic salts. Chem. Eur. J. 2012, 18, 987–994. [Google Scholar] [CrossRef]
- Naud, D.L.; Hiskey, M.A.; Harry, H.H. Synthesis and explosive properties of 5,5'-dinitro-3,3'-azo-1H-1,2,4-triazole (DNAT). J. Energ. Mater. 2003, 21, 57–62. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapötke, T.M.; Martin, F.A.; Wiedbrauk, S. Nitraminoazoles based on ANTA—A comprehensive study of structural and energetic properties. Eur. J. Inorg. Chem. 2012, 2429–2443. [Google Scholar]
- Joo, Y.H.; Shreeve, J.M. Energetic mono-, di-, and trisubstituted nitroiminotetrazoles. Angew. Chem. 2009, 121, 572–575. [Google Scholar] [CrossRef]
- Joo, Y.; Shreeve, J.M. High-density energetic mono- or bis(oxy)-5-nitroiminotetrazoles. Angew. Chem. Int. Ed. 2010, 49, 7320–7323. [Google Scholar] [CrossRef]
- Wang, R.; Xu, H.; Guo, Y.; Sa, R.; Shreeve, J.M. Bis[3-(5-nitroimino-1,2,4-triazolate)]-based energetic salts: Synthesis and promising properties of a new family of high-density insensitive materials. J. Am. Chem. Soc. 2010, 132, 11904–11905. [Google Scholar] [CrossRef]
- Qi, C.; Li, S.H.; Li, Y.C.; Wang, Y.; Zhao, X.X.; Pang, S.P. Synthesis and promising properties of a new family of high-nitrogen compounds: Polyazido- and polyamino-substituted N,N'-azo-1,2,4-triazoles. Chem. Eur. J. 2012, 18, 16562–16570. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Krumm, B.; Martin, F.A.; Stierstorfer, J. New azidotetrazoles: Structurally interesting and extremely sensitive. Chem. Asian J. 2012, 7, 214–224. [Google Scholar] [CrossRef]
- Hinshaw, J.C.; Edwards, W.W.; George, C.; Gilardi, R. Synthesis of 2,3,4,5-tetranitropyrrole. J. Heterocycl. Chem. 1992, 29, 1721–1724. [Google Scholar] [CrossRef]
- Baryshnikov, A.T.; Erashko, V.I.; Zubanova, N.I.; Ugrak, B.I.; Shevelev, S.A.; Fainzil’berg, A.A.; Laikhter, A.L.; Mel’nikova, L.G.; Semenov, V. Gem-dinitro compounds in organic synthesis. 3. Synthesis of 4-nitro-1,2,3-trizoles from gem-dinitro compounds. Bull. Russ. Acad. Sci. Div. Chem. Sci. 1992, 41, 751–757. [Google Scholar] [CrossRef]
- Koldobskii, G.I.; Soldatenko, D.S.; Gerasimova, E.S.; Khokhryakova, N.R.; Shcherbinin, M.B.; Lebedev, V.P.; Ostrovskii, V.A. Tetrazoles: XXXVI.* Synthesis, structure, and properties of 5-nitrotetrazole. Russ. J. Org. Chem. 1997, 33, 1771–1783. [Google Scholar]
- Duddu, R.; Dave, P.R.; Damavarapu, R.; Gelber, N.; Parrish, D. Synthesis of N-amino- and N-nitramino-nitroimidazoles. Tetrahedron Lett. 2010, 51, 399–401. [Google Scholar]
- Li, S.H.; Pang, S.P.; Li, X.T.; Yu, Y.Z.; Zhao, X.Q. Synthesis of new tetrazene (N-N=N-N)-linked bi(1,2,4-triazole). Chin. Chem. Lett. 2007, 18, 1176–1178. [Google Scholar] [CrossRef]
- Qi, C.; Li, S.H.; Li, Y.C.; Wang, Y.; Chen, X.K.; Pang, S.P. A novel stable high-nitrogen energetic material: 4,4'-Azobis(1,2,4-triazole). J. Mater. Chem. 2011, 21, 3221–3225. [Google Scholar] [CrossRef]
- Li, Y.C.; Qi, C.; Li, S.H.; Zhang, H.J.; Sun, C.H.; Yu, Y.Z.; Pang, S.P. 1,1'-Azobis-1,2,3-triazole: A high-nitrogen compound with stable N8 structure and photochromism. J. Am. Chem. Soc. 2010, 132, 12172–12173. [Google Scholar]
- Yin, P.; Zhang, Q.H.; Zhang, J.H.; Parris, D.A.; Shreeve, J.M. N-Trinitroethylamino functionalization of nitroimidazoles: A new strategy for high performance energetic materials. J. Mater. Chem. A 2013, 1, 7500–7510. [Google Scholar]
- Göbel, G.; Klapötke, T.M. Development and testing of energetic materials: The concept of high densities based on the trinitroethyl functionality. Adv. Funct. Mater. 2009, 19, 347–365. [Google Scholar] [CrossRef]
- Simpson, R.L.; Pagoria, P.F.; Mitchell, A.R.; Coon, C.L. Synthesis, properties and performance of the high explosive ANTA. Prop. Explos. Pyrot. 1994, 19, 174–179. [Google Scholar] [CrossRef]
- Kofman, T.P. 5-Amino-3-nitro-1,2,4-triazole and its derivatives. Russ. J. Org. Chem. 2002, 38, 1231–1243. [Google Scholar] [CrossRef]
- Schmidt, R.D.; Lee, G.S.; Pagoria, P.F.; Mitchell, A.R. Synthesis of 4-amino-3,5-dinitro-1H-pyrazole using vicarious nucleophilic substitution of hydrogen. J. Heterocycl. Chem. 2001, 38, 1227–1230. [Google Scholar] [CrossRef]
- Stierstorfer, J.; Tarantik, K.R.; Klapötke, T.M. New energetic materials: Functionalized 1-ethyl-5-aminotetrazoles and 1-ethyl-5-nitriminotetrazoles. Chem. Eur. J. 2009, 15, 5775–5792. [Google Scholar] [CrossRef]
- Fronabarger, J.W.; Williams, M.D.; Sanborn, W.B.; Bragg, J.G.; Parrish, D.A.; Bichay, M. DBX-1—A lead free replacement for lead azide. Propell Explos. Pyrot. 2011, 36, 541–550. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Radies, H.; Stierstorfer, J.; Tarantik, K.R.; Chen, G.; Nagori, A. Coloring propertiesof various high-nitrogen compounds in pyrotechnic compositions. Propell. Explos. Pyrot. 2010, 35, 213–219. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Amination of energetic anions: High-performing energetic materials. Dalton Trans. 2012, 41, 9451–9459. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Parrish, D.A.; Shreeve, J.M. Derivatives of 5-nitro-1,2,3-2H-triazole–high performance energetic materials. J. Mater. Chem. A 2013, 1, 585–593. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.; Parrish, D.A.; Shreeve, J.M. 4-Chloro-3,5-dinitropyrazole: A precursor for promising insensitive energetic compounds. J. Mater. Chem. A 2013, 1, 2863–2868. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Palysaeva, N.V.; Struchkova, M.I.; Suponitsky, K.Y. A mild and efficient synthesis of 3-hetarylamino-s-tetrazines. Mendeleev Commun. 2012, 22, 302–304. [Google Scholar] [CrossRef]
- Garcia, E.; Lee, K.Y. Structure of 3-amino-5-nitro-1,2,4-triazole. ActaCryst. 1992, C48, 1682–1683. [Google Scholar]
- Janssen, J.W.A.M.; Koeners, H.J.; Kruse, C.G.; Habraken, C.L. Pyrazoles. XII. The preparation of 3(5)-nitropyrazoles by thermal rearrangement of N-nitropyrazoles. J. Org. Chem. 1973, 38, 1777–1782. [Google Scholar] [CrossRef]
- Sikder, A.K.; Geetha, M.; Sarwade, D.B.; Agrawal, J.P. Studies on characterisation and thermal behaviour of 3-amino-5-nitro-1,2,4-triazole and its derivatives. J. Hazard. Mater. 2001, 82, 1–12. [Google Scholar] [CrossRef]
- Garnier, E.; Audoux, J.; Pasquinet, E.; Suzenet, F.; Poullain, D.; Lebret, B.; Guillaumet, G. Easy access to 3- or 5-heteroarylamino-1,2,4-triazines by SNAr, SNH and palladium-catalyzed N-heteroarylations. J. Org. Chem. 2004, 69, 7809–7815. [Google Scholar] [CrossRef]
- Licht, H.H.; Ritter, H. Synthesis and explosive properties of dinitrobitriazole. Propell. Explos. Pyrot. 1997, 22, 333–336. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Cao, Y.L.; Sun, Z.X.; He, J.X. Investigation on synthesis and properties of 5,5'-dinitro-3,3'-azo-1-hydro-1,2,4-triazole (DNAT). Chin.J. Solid Rocket Technol. 2008, 31, 501–503. [Google Scholar]
- Xiong, C.L.; Jia, S.Y.; Wang, X.J.; Wang, B.Z.; Zhang, Y.G. Synthesis and extraction of ammonium salt of 3,5-dinitro-1,2,4-triazole. Fine Chem. Intermed. 2008, 38, 64–66. [Google Scholar]
- Kien, Y.L.; Donald, G.O. Production of the Ammonium Salt of 3,5-Dinitro-1,2,4-triazole by Solvent Extraction. U.S. Patent 4236014 A, 25 November 1980. [Google Scholar]
- Vinogradov, V.M.; Dalinger, L.L.; Gulevskaya, V.L.; Shevelev, S.D. Nitropyrazoles. Russ. Chem. Bull. 1993, 42, 1369–1371. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapötke, T.M. Nitrogen-rich bis-1,2,4-triazoles—A comparative study of structural and energetic properties. Chem. Eur. J. 2012, 18, 16742–16753. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabaté, C.M.; Stierstorfer, J. Neutral 5-nitrotetrazoles: Easy initiation with low pollution. New J. Chem. 2009, 33, 136–147. [Google Scholar] [CrossRef]
- Yu, Z.J.; Bernstein, E.R. Sensitivity and performance of azole-based energetic materials. Phys. Chem. A 2013, 117, 10889–10902. [Google Scholar] [CrossRef]
- Sućeska, M. EXPLO5.05 Program, Zagreb, Croatia, 2011.
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, X.; Qi, C.; Zhang, L.; Wang, Y.; Li, S.; Zhao, F.; Pang, S. Amination of Nitroazoles — A Comparative Study of Structural and Energetic Properties. Molecules 2014, 19, 896-910. https://doi.org/10.3390/molecules19010896
Zhao X, Qi C, Zhang L, Wang Y, Li S, Zhao F, Pang S. Amination of Nitroazoles — A Comparative Study of Structural and Energetic Properties. Molecules. 2014; 19(1):896-910. https://doi.org/10.3390/molecules19010896
Chicago/Turabian StyleZhao, Xiuxiu, Cai Qi, Lubo Zhang, Yuan Wang, Shenghua Li, Fengqi Zhao, and Siping Pang. 2014. "Amination of Nitroazoles — A Comparative Study of Structural and Energetic Properties" Molecules 19, no. 1: 896-910. https://doi.org/10.3390/molecules19010896
APA StyleZhao, X., Qi, C., Zhang, L., Wang, Y., Li, S., Zhao, F., & Pang, S. (2014). Amination of Nitroazoles — A Comparative Study of Structural and Energetic Properties. Molecules, 19(1), 896-910. https://doi.org/10.3390/molecules19010896