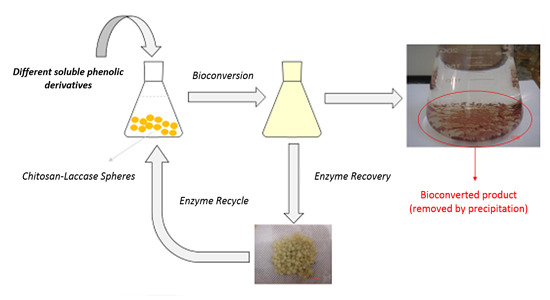
Substrate Specificity and Enzyme Recycling Using Chitosan Immobilized Laccase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzymatic Oxidation of Phenolic Compounds Using Immobilized Laccase
| Phenolic Compound | Molecular Weight(g·mol−1) | % Conversion (Immobilized Enzyme) | % Conversion (Free Enzyme) | ||||
|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 20 °C | 30 °C | 40 °C | ||
| o-Cresol | 108 | 12 ± 2 | 18 ± 5 | 45 ± 3 | 46 ± 3 | 46 ± 9 | 62 ± 2 |
| m-Cresol | 15 ± 5 | 14 ± 2 | 17 ± 2 | 27 ± 1 | 32 ± 10 | 44 ± 7 | |
| p-Cresol | 5 ± 3 | 43 ± 10 | 24 ± 10 | 18 ± 4 | 72 ± 9 | 48 ± 5 | |
| o-Ethylphenol | 122 | 15 ± 8 | 34 ± 7 | 7 ± 1 | 55 ± 1 | 78 ± 7 | 32 ± 3 |
| p-Ethylphenol | 39 ± 7 | 51 ± 3 | 100 ± 0 | 85 ± 7 | 92 ± 8 | 100 ± 0 | |
| 2-Isopropylphenol | 136 | 29 ± 4 | 35 ± 7 | 64 ± 10 | 78 ± 9 | 81 ± 2 | 92 ± 2 |
| 2,3,5-Trimethylphenol | 82 ± 4 | 89 ± 1 | 90 ± 9 | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| 4-Hydroxybenzoic acid | 138 | 27 ± 9 | 32 ± 5 | 35 ± 6 | 40 ± 1 | 62 ± 1 | 80 ± 1 |
| 4-Nitrophenol | 139 | n.d. | n.d. | n.d. | 5 ± 1 | 8 ± 4 | 12 ± 3 |
| 2,3-Xylenol | 122 | 22 ± 10 | 47 ± 6 | 83 ± 1 | 55 ± 6 | 89 ± 2 | 100 ± 0 |
| 2,4-Xylenol | 25 ± 3 | 49 ± 5 | 65 ± 1 | 32 ± 7 | 55 ± 3 | 81 ± 10 | |
| 2,5-Xylenol | 40 ± 1 | 70 ± 1 | 94 ± 3 | 52 ± 00 | 85 ± 4 | 100 ± 0 | |
| 2,6-Xylenol | 65 ± 8 | 92 ± 3 | 97 ± 6 | 91 ± 7 | 100 ± 0 | 100 ± 0 | |
| 3,4-Xylenol | 70 ± 7 | 85 ± 2 | 42 ± 10 | 52 ± 6 | 68 ± 5 | 27 ± 3 | |
| 3,5-Xylenol | 16 ± 3 | 27 ± 9 | 30 ± 7 | 28 ± 5 | 32 ± 9 | 55 ± 3 | |
| Phenol | 94 | 5 ± 1 | 5 ± 2 | 7 ± 2 | 20 ± 3 | 26 ± 1 | 31 ± 7 |
| Syringaldazine | 360 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 2,6-DMP | 154.16 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |


2.2. Kinetic Studies





2.3. Enzyme Reuse and Operational Stability Using Syringaldazine as Substrate

3. Experimental Section
3.1. Chitosan Beads Production and Enzyme Immobilization
3.2. Measurement of Laccase Enzymatic Activity Using Different Phenol Substituents

3.3. Measurement of Phenolic Compounds Concentration
3.4. Measurement of Kinetic Parameters Using Chitosan Immobilized Laccase
3.4.1. Kinetic Parameters Using Syringaldazine as Substrate
3.4.2. Kinetic Parameters Using 2,6-DMP as Substrate

3.5. Evaluation of Enzyme Recyclability
3.6. Evaluation of Enzyme Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peyton, T.O. Biological disposal of hazardous-waste. Enzym. Microb. Technol. 1984, 6, 146–154. [Google Scholar]
- Chen, H.T.; Fredrick, E.E.; Cormack, J.F.; Young, S.R. Four Biological-Systems for Treating Integrated Paper-Mill Effluent. Tappi 1974, 57, 111–115. [Google Scholar]
- Yunusov, T.S.; Yuldashe, P.K. Separation and Purification of Phenoloxydase from Leaves of Cotton Plant. Khimiya Prirodnykh Soedinenii 1974, 122–123. [Google Scholar]
- Benjakul, S.; Visessanguan, W.; Tanaka, M. Properties of phenoloxidase isolated from the cephalothorax of kuruma prawn (Penaeus japonicus). J. Food Biochem. 2005, 29, 470–485. [Google Scholar]
- Bai, G.X.; Brown, J.F.; Watson, C.; Yoshino, T.P. Isolation and characterization of phenoloxidase from egg masses of the gastropod mollusc, Biomphalaria glabrata. Comp. Biochem. Physiol. B 1997, 118, 463–469. [Google Scholar]
- Bornscheuer, U.T. Immobilizing enzymes: How to create more suitable biocatalysts. Angew. Chem. Int. Ed. 2003, 42, 3336–3337. [Google Scholar]
- Betancor, L.; Luckarift, H.R. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008, 26, 566–572. [Google Scholar]
- Whiteley, C.G.; Lee, D.J. Enzyme technology and biological remediation. Enzym. Microb. Technol. 2006, 38, 291–316. [Google Scholar]
- Durán, N.; Espósito, E. Potential applications of oxidative enzymes and phenoloxidases-like compounds in wastewater and soil treatment: A review. Appl. Catal. B 2000, 28, 83–99. [Google Scholar]
- Michniewicz, A.; Ledakowicz, S.; Jamroz, T.; Jarosz–Wilkolazka, A.; Leonowicz, A. Decolorization of aqueous solution of dyes by the laccase in the food Cerrena unicolor. Biotechnologia 2003, 4, 194–203. [Google Scholar]
- Palmieri, G.; Cennamo, G.; Sannia, G. Remazol brilliant blue R decolourisation by the fungus Pleurotus ostreatus and its oxidative enzymatic system. Enzym. Microb. Technol. 2005, 36, 17–24. [Google Scholar]
- Virk, A.P.; Sharma, P.; Capalash, N. Use of laccase in pulp and paper industry. Biotechnol. Prog. 2012, 28, 21–32. [Google Scholar]
- Rodríguez Couto, S.; Hofer, D.; Sanromán, M.A.; Gübitz, G.M. Production of laccase by Trametes hirsuta grown in an immersion bioreactor. Application to decolourisation of dyes from a leather factory. Eng. Life Sci. 2004, 4, 233–238. [Google Scholar]
- D’Annibale, A.; Ricci, M.; Quaratino, D.; Federici, F.; Fenice, M. Panus tigrinus efficiently removes phenols, color and organic load from olive-mill wastewater. Res. Microbiol. 2004, 155, 596–603. [Google Scholar]
- Jaouani, A.; Guillénb, F.; Penninckxa, M.J.; Martínezb, A.T.; Martínezb, M.J. Role of Pycnoporus coccineus laccase in the degradation of aromatic compounds in olive oil mill wastewater. Enzym. Microb. Technol. 2005, 36, 478–486. [Google Scholar]
- Roper, J.; Sarkar, J.M.; Bollag, J.M. Enhanced enzymatic removal of chlorophenols in the presence of co-substrates. Water Res. 1995, 36, 2720–2724. [Google Scholar]
- Zhang, J.; Xua, Z.; Chena, H.; Zonga, Y. Removal of 2,4-dichlorophenol by chitosan-immobilized laccase from Coriolus versicolor. Biochem. Eng. J. 2009, 45, 54–59. [Google Scholar]
- Fernández-Fernández, M.; Sanromán, M.A.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar]
- Sadighi, A.; Faramarzi, M.A. Congo red decolorization by immobilized laccase through chitosan nanoparticles on the glass beads. J. Taiwan Inst. Chem. Eng. 2013, 44, 156–162. [Google Scholar]
- Cabana, H.; Ahamed, A.; Leduc, R. Conjugation of laccase from the white rot fungus Trametes versicolor to chitosan and its utilization for the elimination of triclosan. Bioresour. Technol. 2011, 102, 1656–1662. [Google Scholar]
- D’Annibale, A.; Stazi, S.R.; Vinciguerra, V.; Di Mattia, E.; Sermanni, G.G. Characterization of immobilized laccase from Lentinula edodes and its use in olive-mill wastewater treatment. Process Biochem. 1999, 34, 697–706. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties: Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar]
- Wan, Y.Y.; Du, Y.M.; Miyakoshi, T. Enzymatic catalysis of 2,6-dimethoxyphenol by laccases and products characterization in organic solutions. Sci. China Ser. B Chem. 2008, 51, 669–676. [Google Scholar]
- Woodward, J. Immobilized Enzymes: Adsorption and Covalent Coupling. In Immobilized Cells and Enzymes: A Practical Approach; Woodward, J., Ed.; IRL: Oxford, UK, 1985; pp. 3–17. [Google Scholar]
- Leonowicz, A.; Sarkar, J.M.; Bollag, J.M. Improvement in Stability of an Immobilized Fungal Laccase. Appl. Microbiol. Biotechnol. 1988, 29, 129–135. [Google Scholar]
- Weetall, H.H. Covalent coupling methods for inorganic support materials Methods Enzymol. 1976, 44, 134–148. [Google Scholar]
- Wiseman, A. Handbook of Enzyme Biotechnology; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Xu, F. Oxidation of phenols, anilines and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar]
- Leonowicz, A.; Edgehill, R.U.; Bollang, J.M. The effect of pH on the transformation of syringic and vanillic acids by the laccases of Rhizoctonia praticola and Trametes versicolor. Arch. Microbiol. 1984, 137, 89–96. [Google Scholar]
- Kersten, P.J.; Kalyanaraman, B.; Hammel, K.E.; Reinhammar, B.; Kirk, T.K. Comparisom of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem. J. 1990, 268, 475–480. [Google Scholar]
- Margot, J.; Maillard, J.; Rossi, L.; Barry, D.A.; Holliger, C. Influence of treatment conditions on the oxidation of micropollutants by Trametes versicolor laccase. New Biotechnol. 2013, 30, 803–813. [Google Scholar]
- Felici, M.; Artemi, F.; Luna, M. Determination of laccase activity with various aromatic substrates by high-performance liquid chromatography. J. Chromatogr. A 1985, 320, 435–439. [Google Scholar]
- Morrison, R.T.; Boyd, R.N. Organic Chemistry; Prentice-Hall: Upper Saddle River, NJ, USA, 1992. [Google Scholar]
- Harkin, J.M.; Obst, J.R. Syringaldazine, an effective reagent for detecting laccase and peroxidase in fungi. Experientia 1973, 29, 381–387. [Google Scholar]
- Madhavi, V.; Lele, S.S. Laccase: Properties and applications. Bioresources 2009, 4, 1694–1717. [Google Scholar]
- Farnet, A.M.; Criquet, S.; Tagger, S.; Gil, G.; Le Petit, J. Purification, partial characterization, and reactivity with aromatic compounds of two laccases from Marasmius quercophilus strain 17. Can. J. Microbiol. 2000, 46, 189–194. [Google Scholar]
- Kunamneni, A.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Laccases and Their Applications: A Patent Review. Recent Pat. Biotechnol. 2008, 2, 10–24. [Google Scholar]
- Jiang, D.S.; Long, S.Y.; Huang, J.; Xiao, H.Y.; Zhou, J.Y. Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 2005, 25, 15–23. [Google Scholar]
- Rosa, M.A. Tratamento de efluente de indústria papeleira por processo combinado químico (ozonização) e enzimático (lacase). Doctoral Thesis, Instituto de Química, Universidade de Campinas, Campinas-SP, Brazil, March 2004. [Google Scholar]
- Skoronski, E.; Fernandes, M.; Furigo, A., Jr.; Soares, C.H.L.; João, J.J. Immobilization of laccase (Aspergillus sp.) on chitosan and its application in the bioconversion of phenols in packed bed reactors. Quim. Nova 2014, 37, 215–220. [Google Scholar]
- Morita, T.; Assumpção, R.W.V. Manual de Soluções,Reagentes e Solventes; Edgard Blüchner: São Paulo, Brazil, 1995. [Google Scholar]
- American Public Health Association. In Standard Methods: for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005.
- Majcherczyk, A.; Johannes, C.; Hüttermann, A. Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzym. Microb. Technol. 1998, 22, 335–341. [Google Scholar]
- Pacheco, S.M.V.; Soares, C.H.L. Immobilization and characterization of laccase and its use in the biodegradation of paper mill effluent. Quim. Nova 2014, 37, 209–214. [Google Scholar]
- Sample Availability: Samples are not available from authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoronski, E.; Fernandes, M.; Magalhães, M.D.L.B.; Da Silva, G.F.; João, J.J.; Soares, C.H.L.; Júnior, A.F. Substrate Specificity and Enzyme Recycling Using Chitosan Immobilized Laccase. Molecules 2014, 19, 16794-16809. https://doi.org/10.3390/molecules191016794
Skoronski E, Fernandes M, Magalhães MDLB, Da Silva GF, João JJ, Soares CHL, Júnior AF. Substrate Specificity and Enzyme Recycling Using Chitosan Immobilized Laccase. Molecules. 2014; 19(10):16794-16809. https://doi.org/10.3390/molecules191016794
Chicago/Turabian StyleSkoronski, Everton, Mylena Fernandes, Maria De Lourdes Borba Magalhães, Gustavo Felippe Da Silva, Jair Juarez João, Carlos Henrique Lemos Soares, and Agenor Fúrigo Júnior. 2014. "Substrate Specificity and Enzyme Recycling Using Chitosan Immobilized Laccase" Molecules 19, no. 10: 16794-16809. https://doi.org/10.3390/molecules191016794
APA StyleSkoronski, E., Fernandes, M., Magalhães, M. D. L. B., Da Silva, G. F., João, J. J., Soares, C. H. L., & Júnior, A. F. (2014). Substrate Specificity and Enzyme Recycling Using Chitosan Immobilized Laccase. Molecules, 19(10), 16794-16809. https://doi.org/10.3390/molecules191016794