Aroma-Active Compounds in Jinhua Ham Produced With Different Fermentation Periods
Abstract
:1. Introduction
2. Results and Discussion
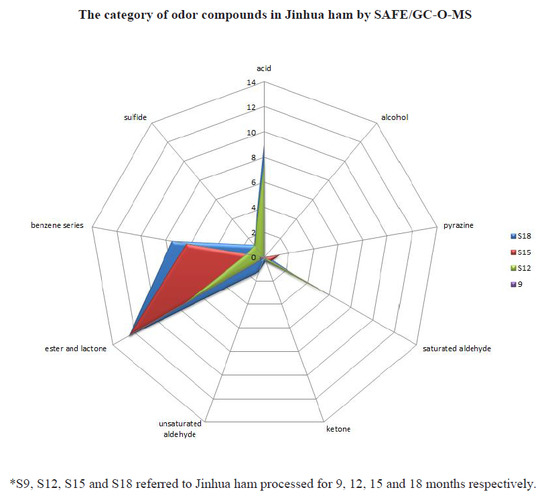
2.1. Aroma-Active Compounds
2.2. Key Aroma-Active Compounds by Dynamic Headspace Dilution Analysis (DHDA)




| Nr a | Compound Name b | R.I. c | Identification d | Odor Property e | FD f | ||||
|---|---|---|---|---|---|---|---|---|---|
| R.I.(DB-5ms) | R.I.(DB-WAX) | 18 | 15 | 12 | 9 c | ||||
| 38 | methanthiol | - | 627 | RI,O | rotten egg | 25 | 125 | 1 | - |
| 76 | trimethylamine | 503 | 848 | RI,O,MS | fishy | 125 | 125 | 125 | 25 |
| 50 | ethyl acetate | 610 | 877 | RI,O,MS,STD | fruity/sweet | 25 | 25 | 1 | - |
| 2 | 3-methylbutanal | 638 | 927 | RI,O,MS | chocolate/malty | 25 | 125 | 5 | 1 |
| 27 | ethanol | - | 941 | RI,O,MS,STD | alcohol | 1 | 1 | - | - |
| 77 | triethylamine | 677 | 970 | RI,O,MS | fishy | 25 | 25 | - | - |
| 17 | 2-pentanone | - | 971 | RI,O,MS | fruity | 5 | 25 | - | - |
| 3 | pentanal | 700 | 984 | RI,O,MS | fermented/yoghourt | 25 | 5 | 5 | - |
| 54 | 2-methyl-butanoic acid ethyl ester | 850 | 1051 | RI,O,MS | fruity/sweet | 25 | 5 | 5 | 1 |
| 51 | acetic acid butyl ester | 812 | 1059 | RI,O | green/fruity | 5 | 5 | - | - |
| 18 | 2-methyl-3-pentanone | - | 1068 | RI,O,MS | mint | 25 | 5 | - | - |
| 61 | disulfide, dimethyl | 750 | 1079 | RI,O,MS | cooked cabbage/onion | 25 | 25 | 1 | 1 |
| 4 | hexanal | 803 | 1094 | RI,O,MS,STD | cut-grass | 125 | 125 | 25 | 5 |
| 32 | 1-methoxy-2-propanol | - | 1137 | RI,O,MS | plastic | 1 | 1 | 1 | 1 |
| 33 | 1-penten-3-ol | 688 | 1164 | RI,O,MS,STD | buttery/grassy/green | 5 | 1 | - | - |
| unknown | - | 1179 | RI,O,MS | popcorn | 5 | 25 | - | - | |
| 5 | heptanal | 905 | 1183 | RI,O,MS | oily/green | 25 | 5 | 1 | - |
| 78 | D-limonene | 1028 | 1191 | RI,O,MS,STD | sweet /orange | 5 | 25 | 5 | - |
| 79 | pyridine | 757 | 1193 | RI,O,MS | spicy | 1 | 1 | 1 | 1 |
| unknown | - | 1199 | O | cooked potato | 25 | 125 | 1 | - | |
| 30 | 3-methyl-1-butanol | - | 1201 | RI,O,MS | fermented/oily/fruity | 5 | - | - | - |
| 80 | 2-pentylfuran | 995 | 1231 | RI,O,MS,STD | fruity/green | 5 | 5 | - | - |
| 52 | hexanoic acid ethyl ester | 1000 | 1232 | RI,O,MS | fruity/apple | - | - | 5 | 1 |
| 28 | 1-pentanol | 760 | 1250 | RI,O,MS | green | 1 | 5 | - | 1 |
| 10 | (E)-2-hexenal | 855 | - | RI,O,MS | green/fatty | 5 | 5 | - | - |
| unknown | - | 1270 | O | popcorn | 125 | 125 | 5 | - | |
| 6 | octanal | 1009 | 1287 | RI,O,MS | fatty | 5 | 5 | 1 | 1 |
| 22 | 3-octen-2-one | 1045 | 1285 | RI,O,MS | fatty/nutty/spicy | 25 | 125 | - | - |
| 20 | 3-hydroxy-2-butanone | 722 | 1286 | RI,O,MS | buttery/green | 5 | 5 | 1 | - |
| 21 | 1-octen-3-one | 980 | 1297 | RI,O,MS,STD | mushroom | 125 | 125 | 5 | 5 |
| 19 | 1-hydroxy-2-propanone | 680 | 1307 | RI,O,MS | nutty/bitter | - | - | 1 | 5 |
| 11 | (E)-2-heptenal | 961 | 1317 | RI,O,MS,STD | fatty/fruity | 25 | 25 | - | - |
| 81 | 2-acetyl-1-pyrroline | 912 | 1339 | RI,O,STD | popcorn | 625 | 125 | 125 | 25 |
| unknown | - | 1345 | O | fishy | 25 | 25 | - | - | |
| 73 | 2,6-dimethylpyrazine | 919 | 1337 | RI,O,MS,STD | toast/nutty | 125 | 125 | 25 | 5 |
| 63 | dimethyl trisulfide | - | 1380 | RI,O,MS | garlic/cooked cabbage | 125 | 25 | 5 | - |
| 74 | trimethylpyrazine | 1006 | 1395 | RI,O,MS,STD | nutty/chocolate | 25 | 5 | 1 | 1 |
| 23 | 1-nonen-3-one | - | 1404 | RI,O,MS | mushroom | 25 | 5 | 1 | - |
| 7 | nonanal | 1102 | 1408 | RI,O,MS | green/fatty/soapy | 25 | 1 | 5 | - |
| 36 | 3,5-octadien-2-ol | - | 1408 | RI,O,MS | green | 1 | - | - | - |
| 12 | (E)-2-octenal | 1064 | 1425 | RI,O,MS | fatty | 25 | 5 | - | - |
| 75 | tetramethylpyrazine | 1084 | - | RI,O,MS,STD | nutty | 1 | - | - | 1 |
| 34 | 1-octen-3-ol | 986 | 1445 | RI,O,MS | mushroom | 5 | 25 | 25 | 25 |
| 39 | acetic acid | 658 | 1446 | RI,O,MS,STD | sour | 125 | 125 | 25 | 25 |
| 9 | methional | 906 | 1450 | RI,O | cooked potato | 125 | 125 | 25 | - |
| 8 | furfural | - | 1453 | RI,O,STD | sweet popcorn/wood | 125 | 25 | - | - |
| 26 | camphor | 1145 | 1503 | RI,O,MS | camphor | 5 | 1 | 1 | - |
| 47 | 2-methylpropanoic acid | 828 | 1550 | RI,O,MS | sock/stinky | 25 | 125 | 1 | 1 |
| 66 | benzaldehyde | 969 | 1515 | RI,O,MS | almond | 5 | 5 | - | - |
| 24 | (E,E)-3,5-octadien-2-one | 1091 | 1520 | RI,O,MS | fatty | 5 | - | 1 | - |
| 40 | propanoic acid | 718 | 1556 | RI,O,MS | sour | 25 | 25 | - | - |
| unknown | - | 1559 | O | chocolate | 25 | 1 | 1 | - | |
| 37 | 2,3-butanediol | 783 | 1581 | RI,O,MS | fruity/creamy/oily | - | - | 5 | 25 |
| 14 | (E,Z)-2,6-nonadienal | - | 1586 | RI,O | cucumber | 1 | 1 | 1 | - |
| 35 | (E)-2-octen-1-ol | - | 1607 | RI,O,MS | mushroom | - | 5 | - | - |
| 55 | butyrolactone | 950 | 1613 | RI,O,MS | hay | 5 | 25 | 1 | - |
| unknown | - | 1617 | O | rancid/fishy | 125 | 125 | - | - | |
| 41 | butanoic acid | 821 | 1630 | RI,O,MS | cheesy | 125 | 125 | 25 | 5 |
| 56 | γ-pentalactone | - | 1632 | RI,O,MS | creamy | 25 | 1 | - | - |
| 53 | decanoic acid, ethyl ester | - | 1637 | RI,O,MS | fruity | 5 | 1 | - | 1 |
| 67 | acetophenone | 1078 | 1612 | RI,O,MS | flower/sweet | - | - | 1 | 1 |
| 48 | 3-methylbutanoic acid | 876 | 1667 | RI,O,MS | sour/sweat | 625 | 625 | 625 | 125 |
| 13 | (E,E)-2,4-nonadienal | 1155 | 1703 | RI,O,MS | fatty/fried | 5 | 1 | - | - |
| 57 | γ-hexanolactone | 1062 | 1705 | RI,O,MS | hay/sweet | 25 | 1 | - | - |
| 42 | pentanoic acid | 911 | 1714 | RI,O,MS | meat/rancid | 125 | 25 | 1 | 1 |
| 65 | 2-acetyl-2-thiazoline | - | 1759 | RI,O | popcorn | 1 | 5 | 5 | - |
| 15 | (E,E)-2,4-decadienal | - | 1812 | RI,O,MS,STD | fatty/fried | 5 | - | 1 | - |
| 43 | hexanoic acid | 997 | 1833 | RI,O,MS | sour/rancid | 5 | 1 | - | - |
| 71 | 4-methylguaiacol | - | 1938 | RI,O | mushroom/smoke | 25 | 5 | - | - |
| 58 | γ-nonalactone | 1124 | 2023 | RI,O | peachy/sweet | 125 | 125 | 125 | 25 |
| 72 | p-cresol | - | 2031 | RI,O | fecal | 125 | 25 | 25 | 1 |
| 59 | δ-nonalactone | 1363 | 2035 | RI,O,MS | coconut/creamy/sweet | 1 | 25 | 5 | 25 |
| 60 | γ-decalactone | 1370 | 2150 | RI,O | peachy | 125 | 125 | 25 | - |
| unknown | - | 2178 | O | medicine | 25 | 25 | 5 | - | |
| unknown | - | 2185 | O | burnt sugar | 125 | 125 | - | - | |

| Nr a | Compound Name | Odor Property | Threshold (ppb in Water) | 18-inner | 18-inner | 15-inner | 15-inner | 12-inner | 12-inner | 9-inner | 9-inner |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concn (ppb) | OAV | Concn (ppb) | OAV | Concn (ppb) | OAV | Concn (ppb) | OAV | ||||
| 76 | trimethylamine | fishy | 0.7 | 2280 ± 234 | 3256 | 730 ± 36 | 1043 | 179 ± 35 | 256 | 327 ± 22 | 467 |
| 2 | 3-methylbutanal | chocolate/malty | 0.2 | 2892 ± 373 | 14,458 | 1413 ± 127 | 7066 | 71 ± 28 | 356 | 1121 ± 65 | 5607 |
| 3 | pentanal | fermented/yoghourt | 25 | - | - | 526 ± 33 | 21 | 358 ± 21 | 14 | - | - |
| 61 | dimethyl disulfide | cooked cabbage/onion | 6 | 1358 ± 279 | 226 | 736 ± 61 | 123 | 725 ± 63 | 121 | 41 ± 9 | 7 |
| 4 | hexanal | cut-grass | 4.5 | 2719 ± 252 | 604 | 1414 ± 221 | 314 | 1896 ± 98 | 421 | 152 ± 12 | 34 |
| 5 | heptanal | oily/green | 3 | 297 ± 33 | 100 | 541 ± 26 | 180 | 128 ± 12 | 43 | 9 ± 2 | 3 |
| 78 | D-limonene | sweet/orange-like | 10 | 248 ± 31 | 25 | - | - | 223 ± 49 | 22 | 323 ± 33 | 32 |
| 80 | 2-pentylfuran | fruity/green | 6 | 61 ± 27 | 10 | 529 ± 41 | 88 | 35 ± 10 | 6 | 114 ± 27 | 19 |
| 6 | octanal | fatty | 0.7 | 148 ± 46 | 211 | 421 ± 68 | 602 | 92 ± 15 | 132 | 5 ± 1 | 7 |
| 34 | 1-octen-3-ol | mushroom | 1 | 243 ± 37 | 243 | - | - | - | - | 5 ± 1 | 5 |
| 39 | acetic acid | sour | 22,000 | 52,796 ± 1673 | 2 | 21,837 ± 751 | 1 | - | - | - | - |
| 41 | butanoic acid | cheesy | 240 | 3167 ± 121 | 13 | 1887 ± 189 | 8 | 1864 ± 149 | 8 | - | - |
| 48 | 3-methylbutanoic acid | sour/sweaty | 120 | 2842 ± 336 | 24 | 1533 ± 267 | 13 | 2601 ± 259 | 22 | - | - |
| Nr a | Compound Name b | R.I. c | Identification d | Odor Property e | Log3FD f | ||||
|---|---|---|---|---|---|---|---|---|---|
| R.I.(DB-5ms) | R.I.(DB-WAX) | 18-inner | 15-inner | 12-inner | 9-inner c | ||||
| 1 | methylpropanal | 540 | 867 | RI,O,MS | green/floral | - | - | <1 | <1 |
| 50 | ethyl acetate | 605 | 880 | RI,O,MS,STD | fruity/sweet | - | 2 | - | 2 |
| 16 | 2-butanone | 603 | 900 | RI,O,MS | green | 2 | - | - | - |
| 27 | ethanol | - | 930 | RI,O,MS,STD | alcohol | - | - | 1 | 1 |
| unknown | - | - | - | floral/sweet | 1 | 1 | - | 3 | |
| 54 | 2-methyl-butanoic acid ethyl ester | 855 | 1049 | RI,O,MS | fruity/sweet | 2 | - | - | 2 |
| 61 | dimethyl disulfide | 750 | 1079 | RI,O,MS | cooked cabbage/onion | 1 | 4 | <1 | <1 |
| 4 | hexanal | 790 | 1084 | RI,O,MS,STD | cut-grass | 4 | 3 | 2 | 1 |
| 80 | 2-pentylfuran | 990 | 1240 | RI,O,MS,STD | fruity/green | 1 | 3 | 2 | <1 |
| 52 | hexanoic acid ethyl ester | 999 | 1232 | RI,O,MS | fruity/apple | - | <1 | - | <1 |
| 62 | isopropyl disulfide | 1018 | - | RI,O,MS | sulfurous | 2 | 1 | 1 | - |
| 20 | 3-hydroxy-2-butanone | 720 | 1286 | RI,O,MS | buttery/green | 1 | - | - | <1 |
| 6 | octanal | 1001 | 1280 | RI,O,MS | fatty | 3 | 2 | 2 | <1 |
| 21 | 1-octen-3-one | - | 1289 | RI,O,STD | mushroom | 3 | 3 | 3 | 2 |
| 73 | 2,6-dimethylpyrazine | 922 | 1308 | RI,O,MS,STD | toast/nutty | 3 | 4 | 2 | 1 |
| 29 | 1-hexanol | 869 | 1360 | RI,O,MS | leafy/green | 1 | 1 | - | - |
| 63 | dimethyl trisulfide | - | 1377 | RI,O,MS | garlic/cooked cabbage | 4 | 1 | 4 | - |
| 7 | nonanal | 1102 | 1385 | RI,O,MS | green/fatty/soapy | 2 | 2 | 2 | 1 |
| 39 | acetic acid | 625 | 1450 | RI,O,MS,STD | sour | 4 | 2 | 3 | 3 |
| 9 | methional | - | 1455 | RI,O | cooked potato | 4 | 3 | 5 | 3 |
| 31 | 2-ethyl-1-hexanol | - | 1487 | RI,O,MS | green | 1 | 1 | - | - |
| 40 | propanoic acid | 668 | 1523 | RI,O,MS | sour | 1 | <1 | 1 | 1 |
| 64 | dipropyl trisulfide | 1104 | 1536 | RI,O,MS | garlic/onion/penetrating | <1 | 2 | - | - |
| 37 | 2,3-butanediol | 800 | 1583 | RI,O,MS | fruity/creamy/oily | <1 | 1 | 2 | 1 |
| 47 | 2-methyl-propanoic acid | 790 | 1501 | RI,O,MS | sock/stinky | 2 | - | - | 1 |
| 41 | butanoic acid | 815 | 1621 | RI,O,MS | cheesy | 4 | 4 | 4 | 4 |
| 48 | 3-methyl-butanoic acid | 848 | 1664 | RI,O,MS | sour/sweat | 5 | 5 | 5 | 4 |
| 42 | pentanoic acid | 933 | 1719 | RI,O,MS | meaty/rancid | 2 | 3 | 1 | 1 |
| 15 | (E,E)-2,4-decadienal | - | 1812 | RI,O,MS,STD | fatty/fried | 4 | 2 | 2 | - |
| 43 | hexanoic acid | 997 | 1856 | RI,O,MS | sour/rancid | 3 | 2 | 2 | - |
| 68 | phenylethyl alcohol | - | 1895 | RI,O,MS | rosy | 1 | - | 1 | - |
| 44 | heptanoic acid | 1076 | 1932 | RI,O,MS | sour | 1 | 1 | - | - |
| 58 | γ-nonalactone | - | 2035 | RI,O,MS | peachy/sweet | 5 | 5 | 5 | 2 |
| 45 | octanoic Acid | 1186 | 2040 | RI,O,MS | sour | 1 | 1 | 2 | 1 |
| 72 | p-cresol | 1084 | 2056 | RI,O,MS | fecal | 4 | 2 | 3 | 3 |
| 25 | 3,6-dimethyl-octan-2-one | - | 2078 | RI,O,MS | fatty | 1 | - | - | - |
| 60 | γ-decalactone | - | 2150 | RI,O | peachy | 5 | 5 | 4 | 3 |
| 46 | decanoic acid | 1382 | 2259 | RI,O,MS | smoky/acid | 1 | - | 2 | <1 |
| 49 | 3-(methylthio)-propanoic acid | - | 2293 | RI,O,MS | oily/acid | 2 | 1 | - | - |
| 69 | benzoic acid | - | 2453 | RI,O,MS | benzoin/balsam | 1 | 2 | - | - |
| 70 | benzeneacetic acid | - | 2562 | RI,O,MS | rosy | 5 | 5 | 4 | 2 |
| Nr a | Compound Name | Odor Property | Threshold (ppb in Water) | 18-inner Concn (ppb) | 18-inner OAV | 15-inner Concn (ppb) | 15-inner OAV | 12-inner Concn (ppb) | 12-inner OAV | 9-inner Concn (ppb) | 9-inner OAV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | hexanal | cut-grass | 4.5 | 986 ± 78 | 219 | 122 ± 9 | 27 | 112 ± 12 | 25 | - | - |
| 6 | octanal | fatty | 0.7 | 264 ± 19 | 377 | 48 ± 6 | 68 | 92 ± 9 | 131 | - | - |
| 73 | 2,6-dimethylpyrazine | toast/nutty | 200 | 206 ± 11 | 1 | 32 ± 6 | 0 | - | - | - | - |
| 7 | nonanal | green/fatty/soapy | 1 | 439 ± 12 | 439 | 261 ± 20 | 261 | 497 ± 26 | 497 | 53 ± 3 | 53 |
| 41 | butanoic acid | cheesy | 240 | 2437 ± 121 | 10 | 978 ± 34 | 4 | 736 ± 31 | 3 | 228 ± 13 | 1 |
| 48 | 3-methylbutanoic acid | sour/sweaty | 120 | 8783 ± 362 | 73 | 6199 ± 531 | 52 | 466 ± 21 | 4 | 1456 ± 98 | 12 |
| 15 | (E,E)-2,4-decadienal | fatty/fried | 0.07 | 489 ± 23 | 6980 | 306 ± 12 | 4371 | 10,971 ± 512 | 768 | - | - |
| 58 | γ-nonalactone | peachy/sweet | 30 | 493 ± 11 | 16 | - | - | 33 ± 7 | 1 | - | - |
| 72 | p-cresol | fecal | 55 | 221 ± 25 | 4 | 127 ± 23 | 2 | 77 ± 11 | 1 | - | - |
2.3. Key Aroma-Active Compounds by Solvent-Assisted Flavor Evaporation (SAFE) and Aroma Extract Dilution Analysis (AEDA)


3. Experimental Section
3.1. Materials and Chemicals
3.2. Preparation of Jinhua Ham Samples
3.3. The Extraction of Aroma Compounds by Dynamic Headspace Sampling (DHS)
3.4. Dynamic Headspace Dilution Analysis (DHDA)
3.5. The Extraction of Aroma Compounds by Solvent-Assisted Flavor Evaporation (SAFE)
3.6. Aroma Extract Dilution Analysis (AEDA)
3.7. Gas Chromatography-Olfactometry-Mass Spectrometry (GC-O-MS) Analysis
3.8. Identification and Quantification of Volatile Compounds
3.9. Odor Activity Value (OAV)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| DHS | dynamic headspace sampling |
| SAFE | solvent-assisted flavor evaporation |
| P&T | purge-and-trap |
| SPME | solid phase microextraction |
| GC-O-MS | gas chromatography-olfactometry- mass spectrometry |
| LRI | linear retention indices |
| AEDA | aroma extract dilution analysis |
| DHDA | dynamic headspace dilution analysis |
| STD | standard deviation |
| TDSA | thermal desorption system autosampler |
| CIS | cold injection system |
| PCA | principal component analysis |
Conflicts of Interest
References
- Morales, R.; Guerrero, L.; Aguiar, A.P.S.; Guardia, M.D.; Gou, P. Factors affecting dry-cured ham consumer acceptability. Meat Sci. 2013, 95, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.; Devaud, S.; Fay, L.B.; Cerny, C.; Steiner, M.; Zurbriggen, B. Aroma-active compounds of dry-cured meat: Italian-type salami and Parma ham. ACS Symp. Ser. 2001, 794, 9–20. [Google Scholar]
- García, C.; Berdagué, J.J.; Antequera, T.; López-Bote, C.; Córdoba, J.J.; Ventanas, J. Volatile components of dry cured Iberian ham. Food Chem. 1991, 41, 23–32. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; Ventanas, J.; García, C. Characterization of the most aroma-active compounds of Iberian ham headspace. J. Agric. Food Chem. 2002, 50, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Song, H.L.; Cadwallader, K.R. Aroma components of American country ham. J. Food Sci. 2008, 73, C29–C35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.H.; Zhao, G.M. History and heritage of Jinhua ham. Anim. Front. 2012, 4, 62–67. [Google Scholar] [CrossRef]
- Zhou, G.H.; Zhao, G.M. Biochemical changes during processing of traditional Jinhua ham. Meat Sci. 2007, 77, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Song, H.L. Key odorants of Jinhua ham headspace and their formation pathway. J. Chin. Inst. Food Sci. Technol. 2006, 6, 48–52. [Google Scholar]
- Du, M.; Ahn, D.U. Volatile substances of Chinese traditional Jinhua ham and Cantonese sausage. J. Food Sci. 2001, 66, 827–831. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, G.; Zhao, G.; Xu, X.; Peng, Z. Changes in flavor compounds of dry-cured Chinese Jinhua ham during processing. Meat Sci. 2005, 71, 291–299. [Google Scholar] [CrossRef]
- Bolzoni, L.; Barbieri, G.; Virgili, R. Changes in volatile compounds of Parma ham during maturation. Meat Sci. 1996, 43, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Ventanas, J.; Cava, R.; Andrés, A.; Garcı́a, C. Volatile compounds of dry-cured Iberian ham as affected by the length of the curing process. Meat Sci. 1999, 52, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Carrapiso, A.I.; Jurado, Á.; Timón, M.L.; Garcı́a, C. Aroma-active compounds of Iberian hams with different aroma characteristics. J. Agric. Food Chem. 2002, 50, 6453–6458. [Google Scholar] [CrossRef] [PubMed]
- Timón, M.L.; Ventanas, J.; Martin, L.; Tejeda, J.F.; Garcı́a, C. Volatile compounds in supercritical carbon dioxide extracts of Iberian ham. J. Agric. Food Chem. 1998, 46, 5143–5150. [Google Scholar] [CrossRef]
- Martin, L.; Timón, M.L.; Petrón, M.J.; Ventanas, J.; Antequera, T. Evolution of volatile aldehydes in Iberian ham matured under different processing conditions. Meat Sci. 2000, 54, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Jurado, Á.; García, C.; Timón, M.L.; Carrapiso, A.I. Effect of ripening time and rearing system on amino acid-related flavour compounds of Iberian ham. Meat Sci. 2007, 75, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation — a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Gallardo, E.; León-Camacho, M. Chemical changes in volatile aldehydes and ketones from subcutaneous fat during ripening of Iberian dry-cured ham. Prediction of the curing time. Food Res. Int. 2014, 55, 381–390. [Google Scholar] [CrossRef]
- Flores, M.; Grimm, C.C.; Toldra, F.; Spanier, A.M. Correlations of sensory and volatile compounds of Spanish “Serrano” dry-cured ham as a function of two processing times. J. Agric. Food Chem. 1997, 45, 2178–2186. [Google Scholar] [CrossRef]
- Stahnke, L.H. Dried sausages fermented with Staphylococcus xylosus at different temperatures and with different ingredient levels—Part I. Chemical and bacteriological data. Meat Sci. 1995, 41, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.; Bolzoni, L.; Parolari, G.; Virgili, R.; Buttini, R.; Careri, M.; Mangia, A. Flavor compounds of dry-cured ham. J. Agric. Food Chem. 1992, 12, 2389–2394. [Google Scholar] [CrossRef]
- Bruna, J.M.; Hierro, E.M.; de la Hoz, L.; Mottram, D.S.; Fernández, M.; Ordóñez, J.A. The contribution of Penicillium aurantiogriseum to the volatile composition and sensory quality of dry fermented sausages. Meat Sci. 2001, 59, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Buscailhon, S.; Berdague, J.L.; Monin, G. Time-related changes in volatile compounds of lean tissue during processing of French dry-cured ham. J. Agric. Food Chem. 1993, 63, 69–75. [Google Scholar] [CrossRef]
- Larsen, T.O.; Frisvad, J.C. Characterization of volatile metabolites from 47 Penicillium taxa. Mycol. Res. 1995, 10, 1153–1166. [Google Scholar] [CrossRef]
- Jelen, H.; Wasowicz, E. Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev. Int. 1998, 14, 391–426. [Google Scholar] [CrossRef]
- Hinrichsen, L.; Pedersen, S.B. Relationship among flavor, volatile compounds, chemical changes, and microflora in Italian-type dry-cured ham during processing. J. Agric. Food Chem. 1995, 43, 2932–2940. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cadwallader, K.R. Aroma extract dilution analysis of blue crab claw meat volatiles. J. Agric. Food Chem. 1994, 42, 2867–2870. [Google Scholar] [CrossRef]
- The LRI and odour database. Available online: www.odour.org.uk.
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Deutsche Forschungsanstalt für Lebensmittelchemie und Institut für Lebensmittelchemie der TU München: Garching, Germany, 1998. [Google Scholar]
- Van den Dool, H.; Dec Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from are available from the vendor described in this manuscript.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-S.; Liu, J.-B.; Yang, Z.-M.; Song, H.-L.; Liu, Y.; Zou, T.-T. Aroma-Active Compounds in Jinhua Ham Produced With Different Fermentation Periods. Molecules 2014, 19, 19097-19113. https://doi.org/10.3390/molecules191119097
Liu X-S, Liu J-B, Yang Z-M, Song H-L, Liu Y, Zou T-T. Aroma-Active Compounds in Jinhua Ham Produced With Different Fermentation Periods. Molecules. 2014; 19(11):19097-19113. https://doi.org/10.3390/molecules191119097
Chicago/Turabian StyleLiu, Xiao-Sheng, Jian-Bin Liu, Zheng-Mao Yang, Huan-Lu Song, Ye Liu, and Ting-Ting Zou. 2014. "Aroma-Active Compounds in Jinhua Ham Produced With Different Fermentation Periods" Molecules 19, no. 11: 19097-19113. https://doi.org/10.3390/molecules191119097
APA StyleLiu, X. -S., Liu, J. -B., Yang, Z. -M., Song, H. -L., Liu, Y., & Zou, T. -T. (2014). Aroma-Active Compounds in Jinhua Ham Produced With Different Fermentation Periods. Molecules, 19(11), 19097-19113. https://doi.org/10.3390/molecules191119097