Photochemistry of Benzotriazoles: Generation of 1,3-Diradicals and Intermolecular Cycloaddition as a New Route toward Indoles and Dihydropyrrolo[3,4-b]Indoles
Abstract
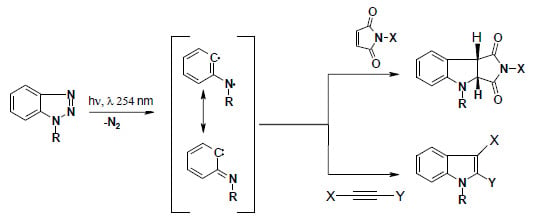
:1. Introduction

2. Results and Discussion


| Run * | Solvent | Time(h) | Molar Ratio (1a):(3a) | Yield (%) |
|---|---|---|---|---|
| 4a | ||||
| 1 | MeOH | 24 | 1:1 | - |
| 2 | DCM | 24 | 1:1 | 5 |
| 3 | THF | 24 | 1:1 | 8 |
| 4 | MePh | 24 | 1:1 | 7 |
| 5 | MeCN | 24 | 1:1 | 12 |
| 6 | MeCN | 24 | 1:2 | 19 |
| 7 | MeCN | 16 | 1:2 | 19 |
| 8 | MeCN | 10 | 1:2 | 12 |
| 9 | MeCN | 36 | 1:2 | 19 |
| Entry * | Reactant | Products (Yield %) | |||||
|---|---|---|---|---|---|---|---|
| 4 | 5 | 6a,b | 7a–c | Unreacted Bt | |||
| 1 | 1a | 3a | 4a (19) | - | 6a(17) | 7a (13) | 45 |
| 2 | 1a | 3b | 4b (21) | - | - | 7b(16) | 58 |
| 3 | 1a | 3c | 4c (19) | - | 6b(18) | 7c(15) | 43 |
| 4 | 1b | 3a | 4d(32) | (20) | - | - | 42 |
| 5 | 1b | 3b | 4e (33) | - | - | - | 60 |
| 6 | 1b | 3c | 4f(35) | - | - | - | 60 |
| 7 | 1c | 3a | 4g (38) | - | - | - | 56 |
| 8 | 1d | 3a | 4h(25) | - | - | - | 70 |
| 9 | 1e | 3a | 4i(35) | - | - | - | 60 |



| Entry | Reactants | Products (Yield %) | |||||
|---|---|---|---|---|---|---|---|
| 9a–f | 10a,b | 11a,b | 12 | Unreacted Bt | |||
| 1 | 1a | 8a | 9a (31) | - | - | - | 60 |
| 2 | 1a | 8b | 9b (36) | - | - | - | 48 |
| 3 | 1a | 8c | 9c (37) | - | - | - | 50 |
| 4 | 1a | 8d | - | 10a (25) | 11a (29) | 12 (19) | 5 |
| 5 | 1a | 8e | - | 10b (31) | 11b (28) | - | 25 |
| 6 | 1b | 8d | 9d (32) | - | - | - | 55 |
| 7 | 1b | 8e | 9e (23) | - | - | - | 61 |
| 8 | 1b | 8c | 9f (19) | - | - | - | 70 |
3. Experimental Section
3.1. General Information
3.2. Irradiation of Benzotriazoles 1a–e with Maleimides 3a–c orAlkynes 8a–e
3.3. Products from Irradiation of Benzotriazoles 1a–e with Maleimides 3a–c
3.4. Photoproducts from Irradiation of Benzotriazoles 1a,b with Alkynes 8a–e
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Burgess, E.M.; Caithers, R.; McCullagh, L. Photochemical decomposition of 1H-1,2,3-triazole derivatives. J. Am. Chem. Soc. 1968, 90, 1923–1924. [Google Scholar]
- Hubert, A.J. Photochemistry of benzotriazoles. J. Chem. Soc. 1969, 10, 1334–1336. [Google Scholar]
- Wender, P.A.; Cooper, C.B. The Photochemistry of 1-Alkenylbenzotriazoles: Methodology for the synthesis of indoles. Tetrahedron 1986, 42, 2985–2991. [Google Scholar]
- Marky, M.; Schmid, H.; Hansen, H. Photoreaktionen von 1-Alkylbenztriazolen. Helv. Chim. Acta 1979, 62, 2129–2153. [Google Scholar]
- Döpp, D.; Orlewska, C.; Saczewski, F. The photolysis of 1-(4,5-dihydro-1H-imidazol-2-yl)benzotriazole. J. Heterocycl. Chem. 1993, 30, 833–834. [Google Scholar]
- Katritzky, A.R.; Lan, X.; Yang, J.; Denisko, O. Properties and synthetic utility of N-substituted benzotriazoles. Chem. Rev. 1998, 98, 409–548. [Google Scholar]
- Katritzky, A.R.; Rachwal, S. Synthesis of Heterocyclesmediated by benzotriazole. 2. Bicyclic systems. Chem. Rev. 2011, 111, 7063–7120. [Google Scholar]
- Kizka, M.; Dunkin, I.R.; Gebicki, J.; Wang, H.; Wirz, J. The photochemical transformation and tautomeric composition of matrix isolated benzotriazole. J. Chem. Soc. Perkin Trans. 2 2000, 2420–2426. [Google Scholar]
- Wang, H.; Burda, C.; Persy, G.; Wirz, J. Photochemistry of 1H-Benzotriazole in Aqueous Solution: A Photolatent Base. J. Am. Chem. Soc. 2000, 122, 5849–5855. [Google Scholar]
- Ohashi, M.; Tsujimoto, K.; Yonezawa, T. Nature of the intermediates in the photolysis of benzotriazoles. J. Chem. Soc. (Sec. D) Chem. Commun. 1970, 1089–1090. [Google Scholar]
- Kulagowski, J.J.; Mitchell, G.; Moody, C.J.; Rees, C.W. Preparation and rearrangement of 6a-methyl-6aH-benzo[a]carbazole and 11b-methyl-11bH-benzo[c]carbazole. J. Chem. Soc. Chem. Commun. 1985. [Google Scholar] [CrossRef]
- Kulagowski, J.J.; Moody, C.J.; Rees, C.W. Preparation and rearrangement of 6a-methyl-6aH-benzo[a]carbazole and 11b-methyl-11bH-benzo[c]carbazole. J. Chem. Soc. Perkin Trans. 1 1985. [Google Scholar] [CrossRef]
- Razmara, F.; Behjati, B.; Afandilians, N. Zur photolyse des benzotriazols. J. Heterocycl. Chem. 1979, 16, 1641. [Google Scholar]
- Tsujimoto, K.; Ohashi, M.; Yonezawa, T. The Photochemical Decomposition of Benzotriazoles. Bull. Chem. Soc. Jpn. 1972, 45, 515–519. [Google Scholar]
- Al-Jalal, N.A.; Al-Awadi, N.A.; Ibrahim, M.R.; Elnagdi, M.H. The photochemistry of 1-alkenyl-substituted-1,2,3-benzotriazolesleading to formation of indole and fused indole derivatives. Arkivoc 2011, X, 288–297. [Google Scholar]
- Al-Jalal, N.A.; Ibrahim, M.R.; Al-Awadi, N.A.; Elnagdi, M.H. The Photochemistry of benzotriazole derivatives. Part 2: Photolysis of 1-substituted benzotriazolearylhydrazones: New route to phenanthridin-6-yl-2-phenyldiazines. Molecules 2011, 16, 10256–10268. [Google Scholar]
- Di Fabio, R.; Micheli, F.; Alvvaro, G.; Cavanni, P.; Donati, D.; Gagliardi, T.; Fontana, G.; Giovannini, R.; Maffeis, M.; Mingardi, A.; et al. From pyrroles to 1-oxo-2,3,4,9-tetrahydro-1H-β-carbolines: A new class of orally bioavailable mGluR1 antagonists. Bioorg. Med. Chem. Lett. 2007; 17, 2254–2259. [Google Scholar]
- Cichero, E.; Cesarini, S.; Mosti, L.; Fossa, P. CoMFA and CoMSIA analyses on 1,2,3,4-tetrahydropyrrolo[3,4-b]indole and benzimidazole derivatives as selective CB2 receptor agonists. J. Mol. Mod. 2010, 16, 1481–1498. [Google Scholar]
- Kempf, D.J.; Condon, S.L. Synthesis of rigid, heterocyclic dipeptide analogs. J. Org. Chem. 1990, 55, 1390–1394. [Google Scholar]
- Bratton, L.D.; Roth, B.D.; Trivedi, B.K.; Unangst, P.C. Synthesis of novel 3- and 5-substituted indole-2-carboxamides. J. Heterocycl. Chem. 2000, 37, 1103–1108. [Google Scholar]
- Behbehani, H.; Ibrahim, M.R.; Ibrahim, Y.A. Efficient atom economic approaches towards macrocyclic crown diamides via ring-closing metathesis. Tetrahedron Lett. 2002, 43, 6421–6426. [Google Scholar]
- Farion, I.A.; Khaltarov, Z.M.; Kushnarev, D.F.; Rokhin, A.V. Synthesis of benzotriazolylsuccinimides in melt. Russ. Chem. Bull. 2008, 57, 409–411. [Google Scholar]
- Novak, I.; Abu-Iznied, T.; Kovac, B.; Klasine, L. Electronic Structure and Stability of Benzotriazoles. J. Phys. Chem. A 2009, 113, 9751–9756. [Google Scholar]
- Cox, M.; Heidarizadeh, F.; Prager, R. Flash vacuum pyrolysis of N-alkenylbenzotriazolesand N-alkenylisoxazolones. Aust. J. Chem. 2000, 53, 665–671. [Google Scholar]
- Crystallographic data of (excluding structure factor) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication nos. CCDC 1012453 (4c), CCDC 101142 (4d), 1011041 (4g), CCDC 1011694 (5), 1012536 (6a) and 1012450 (11a). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223–336033 or e-mail: [email protected].
- Ymane, Y.; Liu, X.; Hamasaki, A.; Ishida, T.; Haruta, M.; Yokoyama, T.; Tokunaga, M. One-Pot synthesis of indolesand aniline derivatives from nitroarenesunder hydrogenation condition with supported gold nanoparticles. Org. Lett. 2009, 11, 5162–5165. [Google Scholar]
- Liu, Y.; Gribble, G.W. Generation and reactions of 2,3-dilithio-N-methylindole. Synthesis of 2,3-disubstituted indoles. Tetrahedron Lett. 2001, 42, 2949–2951. [Google Scholar]
- Sayyed, I.A.; Alex, K.; Tillack, A.; Schwarz, N.; Michalik, D.; Beller, M. A Convenient and General Method for the Synthesis of Indole-2,3-dicarboxylates and 2-Arylindole-3-carboxylates. Eur. J. Org. Chem. 2007, 4525–4528. [Google Scholar]
- Eggers, M.E.; Jog, P.V.; Bates, D.K. Intramolecularsulfoxide electrophilic sulfenylation in 2- and 3-indoleanilides. Tetrahedron 2007, 63, 12185–12194. [Google Scholar]
- Sample Availability: Samples of the compoundsare available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jalal, N.A.; Ibrahim, M.R.; Al-Awadi, N.A.; Elnagdi, M.H.; Ibrahim, Y.A. Photochemistry of Benzotriazoles: Generation of 1,3-Diradicals and Intermolecular Cycloaddition as a New Route toward Indoles and Dihydropyrrolo[3,4-b]Indoles. Molecules 2014, 19, 20695-20708. https://doi.org/10.3390/molecules191220695
Al-Jalal NA, Ibrahim MR, Al-Awadi NA, Elnagdi MH, Ibrahim YA. Photochemistry of Benzotriazoles: Generation of 1,3-Diradicals and Intermolecular Cycloaddition as a New Route toward Indoles and Dihydropyrrolo[3,4-b]Indoles. Molecules. 2014; 19(12):20695-20708. https://doi.org/10.3390/molecules191220695
Chicago/Turabian StyleAl-Jalal, Nader A., Maher R. Ibrahim, Nouria A. Al-Awadi, Mohamed H. Elnagdi, and Yehia A. Ibrahim. 2014. "Photochemistry of Benzotriazoles: Generation of 1,3-Diradicals and Intermolecular Cycloaddition as a New Route toward Indoles and Dihydropyrrolo[3,4-b]Indoles" Molecules 19, no. 12: 20695-20708. https://doi.org/10.3390/molecules191220695
APA StyleAl-Jalal, N. A., Ibrahim, M. R., Al-Awadi, N. A., Elnagdi, M. H., & Ibrahim, Y. A. (2014). Photochemistry of Benzotriazoles: Generation of 1,3-Diradicals and Intermolecular Cycloaddition as a New Route toward Indoles and Dihydropyrrolo[3,4-b]Indoles. Molecules, 19(12), 20695-20708. https://doi.org/10.3390/molecules191220695