Antiprotozoal Activity against Entamoeba histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia graveolens and Ruta chalepensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Susceptibility Assays of Plant Extracts
| Scientific Name | Family | Voucher Specimen | Part Used a | Yield (%w/w) b |
|---|---|---|---|---|
| Agave lechugilla Torr | Agavaceae | 025529 | L | 16.5 |
| Amphipterygium adstringens Standley | Julianaceae | 025530 | B | 18.8 |
| Apium graveolens Linnaeus | Apiaceae | 025531 | S | 5.7 |
| Arctostaphylos pungens Kunth | Ericaceae | 025532 | FR | 23.3 |
| Artemisia mexicana Willd | Asteraceae | 025533 | L | 15.3 |
| Bougainvillea spectabilis Willd | Nyctaginaceae | 025535 | F | 24.5 |
| Capsicum annuum Linnaeus | Solanaceae | 025536 | FR | 37.9 |
| Castela texana Torr & Grey | Simaroubaceae | 025538 | L | 14.0 |
| Cecropia obtusifolia Bertol | Cecropiaceae | 025539 | AP | 12.6 |
| Coriandrum sativum Linnaeus | Apiaceae | 025540 | L | 31.8 |
| Cyclolepis genistoides Don | Asteraceae | 025541 | L | 16.5 |
| Cymbopogon citrates Stapf | Poaceae | 025542 | L | 17.6 |
| Eryngium heterophyllum Engelm | Apiaceae | 025544 | A | 15.3 |
| Eucalyptus globulus Labill | Myrtaceae | 025545 | L | 10.8 |
| Foeniculum vulgare Miller P. | Apiaceae | 025546 | L | 12.4 |
| Gnaphalium oxyphyllum DC | Asteraceae | 025572 | L | 11.2 |
| Gymnosperma glutinosum Spreng | Asteraceae | 025547 | AP | 39.9 |
| Haematoxylon brasiletto Karsten | Leguminosae | 025548 | L | 18.8 |
| Heterotheca inuloides Cass | Asteraceae | 025549 | AP | 16.3 |
| Hibiscus sabdariffa Linnaeus | Malvaceae | 025550 | F | 44.0 |
| Juglans mollis Engelm | Juglandaceae | 025551 | L | 6.3 |
| Lippia graveolens Kunth | Verbenaceae | 025554 | AP | 41.0 |
| Marrubium vulgare Linnaeus | Lamiaceae | 025555 | AP | 15.6 |
| Melissa officinalis Linnaeus | Lamiaceae | 025557 | L | 17.9 |
| Mentha spicata Crantz | Lamiaceae | 025558 | L | 18.9 |
| Ocimum basilicum Linnaeus | Lamiaceae | 025559 | L | 18.5 |
| Opuntia ficus-indica Linnaeus | Cactaceae | 025560 | CL | 17.0 |
| Persea Americana Mill | Lauraceae | 025563 | L | 21.2 |
| Ruta chalepensis Pers | Rutaceae | 025579 | AP | 12.7 |
| Schinus molle Linnaeus | Anacardiaceae | 025567 | AP | 15.9 |
| Syzygium aromaticum Linnaeus | Myrtaceae | 025569 | F c | 39.9 |
| Tilia platyphyllos Scopoli | Tiliaceae | 025570 | F | 11.8 |
| Plant Specimen | % Growth Inhibition b | IC50 of Crude Extract (µg/mL) b |
|---|---|---|
| Lippia graveolens Kunth | 91.54 | 59.14 |
| Ruta chalepensis Pers | 90.50 | 60.07 |
| Capsicum annuum Linnaeus | 87.87 | 98.75 |
| Opuntia ficus-indica Linnaeus | 87.47 | 70.33 |
| Haematoxylon brasiletto Karsten | 84.84 | 96.38 |
| Schinus molle Linnaeus | 81.79 | 32.45 |
| Melissa officinalis Linnaeus | 76.95 | c |
| Castela texana Torr & Grey | 73.82 | c |
| Cyclolepis genistoides Don | 73.80 | c |
| Juglans mollis Engelm | 71.87 | c |
| Agave lechugilla Torr | 69.66 | c |
| Mentha spicata Crantz | 65.72 | c |
| Tilia platyphyllos Scopoli | 65.00 | c |
| Gymnosperma glutinosum Spreng | 63.80 | c |
| Gnaphalium oxyphyllum DC | 42.15 | c |
| Apium graveolens Linnaeus | 29.03 | c |
| Cecropia obtusifolia Bertol | 29.00 | c |
| Persea americana Mill | 24.65 | c |
2.2. Phytochemistry of Bioactive Plants against Entamoeba histolytica
2.3. Isolation and Structure Elucidation of Compounds with Antiprotozoal Activity

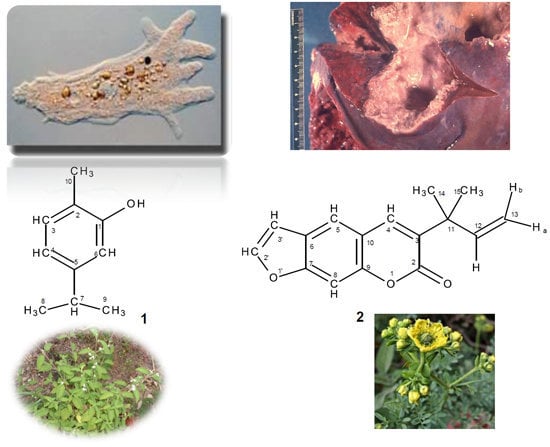
2.3.1. Carvacrol from Lippia graveolens
2.3.2. Chalepensin from Ruta chalepensis
2.4. Possible Antiprotozoal Mechanism of Action of Carvacrol and Chalepensin
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Bioguided Isolation of Carvacrol (1)
3.3.2. Bioguided Isolation of Chalepensin (2)
3.4. Antiprotozoal Assay
3.4.1. Test Microorganisms
3.4.2. In Vitro Assay for Entamoeba histolytica
3.4.3. In Vitro IC50 Determination
4. Conclusions
Acknowledgments
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Kirmizibekmez, H.; Atay, I.; Kaiser, M.; Brun, R.; Cartagena, M.M.; Carballeira, N.M.; Yesilada, E.; Tasdemir, D. Antiprotozoal activity of Melampyrum arvense and its metabolites. Phytother. Res. 2011, 25, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.A.; Petersen, C.A. Chronic infection by Leishmania amazonensis mediated through MAPK ERK mechanisms. Immunol. Res. 2014, 59, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Sosoniuk, E.; Vallejos, G.; Kenawy, H.; Gaboriaud, C.; Thielens, N.; Fujita, T.; Schwaeble, W.; Ferreira, A.; Valck, C. Trypanozoma cruzi calreticulin inhibits the complement lectin pathway activation by direct interaction with L-Ficolin. Mol. Immunol. 2014, 60, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Woc-Colburn, L.; Bottazzi, M.E. Neglected tropical diseases in Central America and Panama: Review of their prevalence, populations at risk and impact on regional development. Int. J. Parasitol. 2014, 44, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Zerpa-Larrauri, R.; Náquira-Velarde, C.; Espinoza, Y. A new vision of Entamoeba histolytica. Rev. Peru. Med. Exp. Salud Publica 2007, 24, 190–192. [Google Scholar]
- López-Camarillo, C.; López-Rosas, I.; Ospina-Villa, J.D.; Marchat, L.A. Deciphering molecular mechanisms of mRNA metabolism in the deep-branching eukaryote Entamoeba histolytica. WIREs RNA 2014, 5, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Sehgal, R.; Chawla, Y.; Malla, N.; Mahajan, R.C. Multidrug resistance in amoebiasis patients. Indian J. Med. Res. 2006, 124, 189–194. [Google Scholar]
- Pinilla, A.E.; López, M.C.; Viasus, D.F. History of the Entamoeba histolytica protozoan. Rev. Med. Chile 2008, 136, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ximénez, C.; Morán, P.; Ramos, F.; Ramiro, M. Amibiasis intestinal: Estado actual del conocimiento. Med. Interna Mex. 2007, 23, 398–407. [Google Scholar]
- Calzada, F.; Yépez-Mulia, L.; Aguilar, A. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J. Ethnopharmacol. 2006, 108, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Ximénez, C.; Morán, P.; Rojas, L.; Valadez, A.; Gómez, A. Reassessment of the epidemiology of amebiasis: State of the art. Infect. Gen. Evol. 2009, 9, 1023–1032. [Google Scholar] [CrossRef]
- Abhyankar, M.M.; Shrimal, S.; Gilchrist, C.A.; Bhattacharya, A.; Petri, W.A., Jr. The Entamoeba histolytica serum-inducible transmembrane kinase EhTMKB1–9 is involved in intestinal amebiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D.; Wright, C.W. Antiprotozoal agents from plant sources. Planta Med. 1991, 57, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Somlata; Babuta, M.; Bhattacharya, S.; Bhattacharya, A. Protein kinases of the parasitic protist Entamoeba histolytica. In Protein Phosphorylation in Parasites: Novel Targets for Antiparasitic Intervention; Doerig, C., Spaeth, G., Wiese, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 131–153. [Google Scholar]
- Schlosser, A.; Leitsch, D.; Duchêne, M. Entamoeba histolytica: Identification of thioredoxin-targeted proteins and analysis of serine acetyltransferase-1 as a prototype example. Biochem. J. 2013, 451, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Samarawickrema, N.A.; Brown, D.M.; Upcroft, J.A.; Thammapalerd, N.; Upcroft, P. Involvement of superoxide dismutase and pyruvate:ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J. Antimicrob. Chemother. 1997, 40, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, C.; Hellberg, A.; Tannich, E.; Bruchhaus, I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J. Biol. Chem. 1999, 274, 26051–26056. [Google Scholar] [CrossRef] [PubMed]
- Conde-Bonfil, M.D.C.; Mora-Zerpa, C.D.L. Entamoeba histolytica: Un desafío vigente. Salud Pub. Mex. 1992, 34, 335–341. [Google Scholar]
- Bendesky, A.; Menéndez, D. Metronidazol: Una visión integral. Rev. Fac. Med. UNAM 2001, 44, 255–259. [Google Scholar]
- Bautista, E.; Calzada, F.; Ortega, A.; Yépez-Mulia, L. Antiprotozoal activity of flavonoids isolated from Mimosa tenuiflora (Fabaceae-Mimosoidae). J. Mex. Chem. Soc. 2011, 55, 251–253. [Google Scholar]
- Singh, S.; Bharti, N.; Mohapatra, P.P. Chemistry and biology of synthetic and naturally occurring antiamoebic agents. Chem. Rev. 2009, 109, 1900–1947. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kiderlen, A.F.; Croft, S.L. Natural products as parasitic drugs. Parasitol. Res. 2003, 90, S55–S62. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.; Güida, C.; Coussio, J.; Paveto, C.; Muschietti, L.; Martino, V. In vitro evaluation of trypanocidal activity in plants used in Argentine traditional medicine. Parasitol. Res. 2006, 98, 370–374. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Licea, R.; Morado-Castillo, R.; Gomez-Flores, R.; Laatsch, H.; Verde-Star, M.J.; Hernández-Martínez, H.; Tamez-Guerra, P.; Tamez-Guerra, R.; Rodríguez-Padilla, C. Bioassay-guided isolation and identification of cytotoxic compounds from Gymnosperma glutinosum leaves. Molecules 2012, 17, 11229–11241. [Google Scholar] [CrossRef] [PubMed]
- Molina-Garza, Z.J.; Bazaldúa-Rodríguez, A.F.; Quintanilla-Licea, R.; Galaviz-Silva, L. Anti-Trypanozoma cruzi activity of 10 medicinal plants used in northeast Mexico. Acta Trop. 2014, 136, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Sousa, M.C.; Salgueiro, L.; Cavaleiro, C. Effects of essential oils in growth of Giardia lamblia trophozoites. Nat. Prod. Commun. 2010, 5, 137–141. [Google Scholar] [PubMed]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Cavaleiro, C.; Custódio, J.B.A.; Sousa, M.C. Anti-Giardia activity of phenolic-rich essential oils: Effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitol. Res. 2010, 106, 1205–1215. [Google Scholar]
- Rufino-González, Y.; Ponce-Macotela, M.; González-Maciel, A.; Reynoso-Robles, R.; Jiménez-Estrada, M.; Sánchez-Contreras, Á.; Martínez-Godillo, M.N. In vitro activity of the F-6 fraction of oregano against Giardia intestinalis. Parasitology 2012, 139, 434–440. [Google Scholar]
- Machado, M.; Santoro, G.; Sousa, M.C.; Salgueiro, L.; Cavaleiro, C. Activity of essential oils on the growth of Leishmania infantum promastigotes. Flavour Fragr. J. 2010, 25, 156–160. [Google Scholar] [CrossRef]
- Ahmed, S.B.H.; Sghaier, R.M.; Guesmi, F.; Kaabi, B.; Mejri, M.; Attia, H.; Laouini, D.; Smaali, I. Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the leishmaniasis-endemic region of Sned (Tunisia). Nat. Prod. Res. 2011, 25, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, E.; Maes, L.; Salama, M.M. In vitro activities of plant extracts from Saudi Arabia against Malaria, Leishmaniasis, Sleeping Sickness and Chagas Disease. Phytother. Res. 2010, 24, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Malatyali, E.; Tepe, B.; Degerli, S.; Berk, S. In vitro amoebicidal activities of Satureja cuneifolia and Melissa officinalis on Acanthamoeba castellanii cysts and trophozoites. Parasitol. Res. 2012, 110, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Calzado-Flores, C.C.; Segura-Luna, J.J.; Domínguez, X.A.; García-González, S. Castela texana: Cernimiento de su actividad antiamibiana. Arch. Investig. Med. (Mex.) 1986, 17, 127–134. [Google Scholar]
- Calzado-Flores, C.; Verde-Star, J.; Lozano-Garza, G.; Segura-Luna, J.J. Preliminary acute toxicological study of Castela texana. Proc. West. Pharamacol. Soc. 1998, 41, 77–78. [Google Scholar]
- Reyes-López, M.; Villa-Treviño, S.; Arriaga-Alba, M.; Alemán-Lazarini, L.; Rodríguez-Mendiola, M.; Arias-Castro, C.; Fattel-Fazenda, S.; Garza, M.D.L. The amoebicidal aqueous extract from Castela texana possesses antigenotoxic and antimutagenic properties. Toxicol. In Vitro 2005, 19, 91–97. [Google Scholar] [CrossRef] [PubMed]
- González-Güereca, M.C.; Soto-Hernández, M.; Kite, G.; Martínez-Vázquez, M. Antioxidant activity of flavonoids from the stem of the Mexican oregano (Lippia graveolens HBK var. berlandieri schauer). Rev. Fitotec. Mex. 2007, 30, 43–49. [Google Scholar]
- Lin, L.Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Comp. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef]
- Calzada, F.; Meckes, M.; Cedillo-Rivera, R. Antiamoebic and antigardial activity of plant flavonoids. Planta Med. 1999, 65, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; McChesney, J.D.; Sindelar, R.D.; Goins, D.K.; Walker, L.A. A new quassinoid from Castela texana. J. Nat. Prod. 1996, 59, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Lugo, E.C.; Díaz, L.; Villanueva, S. Extracción y cuantificación indirecta de las saponinas de Agave lechuguilla Torrey. E-Gnosis 2005, 3, 1–9. [Google Scholar]
- Romero-González, J.; Peralta-Videa, J.R.; Rodríguez, E.; Delgado, M.; Gardea-Torresdey, J.L. Potential of Agave lechuguilla biomass for Cr(III) removal from aqueous solutions: Thermodynamic studies. Bioresour. Technol. 2006, 97, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Monterrosas-Brisson, N.; Arenas-Ocampo, M.L.; Jiménez-Ferrer, E.; Jiménez-Aparicio, A.R.; Zamilpa, A.; Gonzalez-Cortazar, M.; Tortoriello, J.; Herrera-Ruiz, M. Anti-inflammatory activity of different Agave plants and the compound Cantalasaponin-1. Molecules 2013, 18, 8136–8146. [Google Scholar] [CrossRef] [PubMed]
- Momin, R.A.; Ramsewak, R.S.; Nair, M.G. Bioactive compounds and 1,3-di[(cis)-9 octadecenoyl]-2-[(cis,cis)-9,12-octadecadienoyl]glicerol from Apium Graveolens L. Seeds. J. Agric. Food Chem. 2000, 48, 3785–3788. [Google Scholar] [CrossRef] [PubMed]
- Momin, R.A.; Nair, M.G. Mosquitocidal, nematicidal, and antifungal compounds from Apium graveolens L. Seeds. J. Agric. Food Chem. 2001, 49, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, J.; Ishikawa, T.; Satoh, M. Polar constituents of celery seed. Phytochemistry 2003, 64, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Fazal, S.S.; Ansari, M.M.; Singla, R.K.; Khan, S. Isolation of 3-n-butyl phthalide & sedanenolide from Apium graveolens Linn. Indo Glob. J. Pharm. Sci. 2012, 2, 258–261. [Google Scholar]
- Ochi, T.; Takaishi, Y.; Kogure, K.; Yamauti, I. Antioxidant activity of a new capsaicin derivative from Capsicum annuum. J. Nat. Prod. 2003, 66, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Ochi, T.; Takaishi, Y.; Kawazoe, K.; Lee, K.H. New sesquiterpenes from Capsicum annuum. J. Nat. Prod. 2004, 67, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Acero-Ortega, C.; Dorantes-Alvarez, L.; Hernández-Sánchez, H.; Gutiérrez-López, G.; Aparicio, G.; Jaramillo-Flores, M.E. Evaluation of phenylpropanoids in ten Capsicum annuum L. varieties and their inhibitory effects on Listeria monocytogenes Murray, Webb and Swann Scott A. Food Sci. Tech. Int. 2005, 11, 5–6. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Vazquez, R.C. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. J. Ethnopharm. 2010, 130, 93–97. [Google Scholar] [CrossRef]
- De Heluani, C.S.; de Boggiato, M.V.; Catalán, C.A.N.; Díaz, J.G.; Gédris, T.E.; Herz, W. Triterpenes and sesquiterpene lactones from Cyclolepis genistoides. Phytochemistry 1997, 45, 801–805. [Google Scholar] [CrossRef]
- Sosa, A.; Fusco, M.R.; Rossomando, P.; Juárez, A.; Robles, S.; Petenatti, E.; Pelzer, L. Anti-inflammatory properties from isolated compounds of Cyclolepis genistoides. Pharm. Biol. 2011, 49, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Villagómez-Ibarra, J.R.; Sánchez, M.; Espejo, O.; Zúñiga-Estrada, A.; Torres-Valencia, J.M.; Joseph-Nathan, P. Antimicrobial activity of three Mexican Gnaphalium species. Fitoterapia 2001, 72, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Canales, M.; Hernández, T.; Serrano, R.; Hernández, L.B.; Duran, A.; Ríos, V.; Sigrist, S.; Hernández, H.L.H.; Garcia, A.M.; Angeles-López, O.; et al. Antimicrobial and general toxicity activities of Gymnosperma glutinosum: A comparative study. J. Ethnopharmacol. 2007, 110, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Hernández, T.; Canales, M.; García-Bores, A.M.; Romo-De-Vivar, A.; Céspedes, C.L.; Avila, J.G. Ent-labdane type diterpene with antifungal activity from Gymnosperma glutinosum (Spreng.) Less. (Asteraceae). BLACPMA 2009, 8, 412–418. [Google Scholar]
- Rivero-Cruz, J.F. Antimicrobial compounds isolated from Haematoxylon brasiletto. J. Etnopharmacol. 2008, 119, 99–103. [Google Scholar] [CrossRef]
- Sarer, E.; Kökdil, G. Constituents of the essential oil from Melissa officinalis. Planta Med. 1991, 57, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, Melissa, and Sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.M. Mint: The Genus. In Mentha, 1st ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Saleem, M.; Kim, H.J.; Han, C.K.; Jin, C.; Lee, Y.S. Secondary metabolites from Opuntia ficus-indica var. Saboten. Phytochemistry 2006, 67, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Domergue, F.; Helms, G.L.; Prusky, D.; Browse, J. Antifungal compounds from idioblast cells isolated from avocado fruits. Phytochemistry 2000, 54, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.K.; Murakami, A.; Nakamura, Y.; Takeda, N.; Yoshizumi, H.; Ohigashi, H. Novel nitric oxide and superoxide generation inhibitors, Persenone A and B, from avocado fruit. J. Agric. Food Chem. 2000, 48, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, C.H.; Lee, H.S. Antimicrobial activity of quinoline derivatives isolated from Ruta chalepensis toward human intestinal bacteria. J. Microbiol. Biotechnol. 2005, 15, 646–651. [Google Scholar]
- Emam, A.M.; Swelam, E.S.; Megally, N.Y. Furocoumarin and quinolone alkaloid with larvicidal and antifeedant activities isolated from Ruta chalepensis leaves. I. J. Nat. Prod. 2009, 2, 10–22. [Google Scholar]
- Ono, M.; Yamashita, M.; Mori, K.; Masuoka, C.; Eto, M.; Kinjo, J.; Ikeda, T.; Yoshimitsu, H.; Nohara, T. Sesquiterpenoids, triterpenoids, and flavonoids from the fruits of Schinus molle. Food Sci. Technol. Res. 2008, 14, 499–508. [Google Scholar] [CrossRef]
- Rădulescu, V.; Oprea, E. Analysis of volatile compounds of officinal Tiliae flos by gas–chromatography coupled with mass spectrometry. Farmacia 2008, 61, 129–138. [Google Scholar]
- Toker, G.; Aslan, M.; Yeşilada, E.; Memişoğlu, M.; Ito, S. Comparative evaluation of the flavonoid content in officinal Tiliae flos and Turkish lime species for quality assessment. J. Pharm. Biomed. Anal. 2001, 26, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Chiarabini, L.; Alachkar, A.; Chehna, M.F.; Vincieri, F.F.; Bilia, A.R. HPLC–DAD and HPLC–ESI-MS analyses of Tiliae flos and its preparations. J. Pharm. Biomed. Anal. 2014, 100, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Vega, D.E.; Verde-Star, M.J.; Salinas-González, N.; Rosales-Hernández, B.; Estrada-García, I.; Mendez-Aragón, P.; Carranza-Rosales, P.; González-Garza, M.T.; Castro-Garza, J. Antimycobacterial activity of Juglans regia, Juglans mollis, Carya illinoensis and Bocconia frutescens. Phytother. Res. 2008, 22, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.I.; Sharma, E.; Ahmad, G. Juglans regia Linn: A phytopharmacological review. World J. Pharm. Sci. 2014, 2, 364–373. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ahn, Y.J.; Lee, S.B.; Okubo, T.; Kim, M. Antignawing factor of crude oil derived from Thjopsis dolabrata S. et Z. var. hondais sawdust against mice. J. Chem. Ecol. 1995, 21, 263–271. [Google Scholar] [CrossRef]
- Tang, X.; Chen, S.; Wang, L. Purification and identification of carvacrol from the root of Stellera chamaejasme and research on its insecticidal activity. Nat. Prod. Res. 2011, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Vernin, G.; Lageot, C.; Gaydou, E.M.; Parkanyi, C. Analysis of the essential oil of Lippia graveolens HBK from El Salvador. Flavour Fragr. J. 2001, 16, 21–226. [Google Scholar] [CrossRef]
- Rivero-Cruz, I.; Duarte, G.; Navarrete, A.; Bye, R.; Linares, E.; Mata, R. Chemical composition and antimicrobial and spasmolytic properties of Poliomintha longiflora and Lippia graveolens essential oils. J. Food Sci. 2011, 76, C309–C317. [Google Scholar] [CrossRef] [PubMed]
- Senatore, F.; Rigano, D. Essential oil of two Lippia spp. (Verbenaceae) growing wild in Guatemala. Flavour Fragr. J. 2001, 16, 169–171. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Cavaleiro, C.; Gonçalves, M.J.; Cunha, A.P.D. Antimicrobial activity and chemical composition of the essential oil of Lippia graveolens from Guatemala. Planta Med. 2003, 69, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Hernández, T.; Canales, M.; Duran, A.; García, A.M.; Avila, J.G.; Hernández-Portilla, L.; Alvarado, M.; Romero, M.; Terán, B.; Dávila, P.; et al. Variation in the hexanic extract composition of Lippia graveolens in an arid zone from Mexico: Environmental influence or true chemotypes? Open Plant Sci. J. 2009, 3, 29–34. [Google Scholar] [CrossRef]
- Hernández, T.; Canales, M.; Avila, J.G.; García, A.M.; Meraz, S.; Caballero, J.; Lira, R. Composition and antibacterial activity of essential oil of Lippia graveolens H.B.K. (Verbenaceae). BLACPMA 2009, 8, 295–300. [Google Scholar]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Arfa, A.B.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 14–154. [Google Scholar]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3120. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T.; Zeytinoglu, M. Effects of carvacrol on a human non-small cell lung cancer (NSCLC) cell line, A549. Cytotechnology 2003, 43, 14–154. [Google Scholar] [CrossRef]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Zeytinoglu, H.; Incesu, Z.; Baser, K.H.C. Inhibition of DNA synthesis by carvacrol in mouse myoblast cells bearing a human N-RAS oncogene. Phytomedicine 2003, 10, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.Z.; Lu, C.H. Carvacrol-induced [Ca2+]i rise and apoptosis in human glioblastoma cells. Life Sci. 2012, 90, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.Z.; Chou, C.T.; Lu, T.; Chi, C.C.; Tseng, L.L.; Pan, C.C.; Lin, K.L.; Kuo, C.C.; Jan, C.R. The mechanism of carvacrol-evoked [Ca2+]i rises and non-Ca2+-triggered cell death in OC2 human oral cancer cells. Toxicology 2013, 303, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Marshall, M.R.; Wei, C.I. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Burt, S.A.; Fleddermann, M.J.; Haagsman, H.P.; van Knapen, F.; Veldhuizen, E.J.A. Inhibition of Salmonella enterica serotype Enteritidis on agar and raw chicken by carvacrol vapour. Int. J. Food. Microbiol. 2007, 119, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsehakis, M. Antimicrobial and cytotoxic activities of Origanum essential oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Vale-Silva, L.A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L.; Pinto, E. Antifungal activity of the essential oil of Thymus x viciosoi against Candida, Cryptococcus, Aspergillus and dermatophyte species. Planta Med. 2010, 76, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Zabka, M.; Pavela, R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kahn, A.; Akhtar, F.; Yousuf, S.; Xess, I. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Demirci, F.; Baser, K.H.C. Essential oil of Turkish Origanum onites L. and its main components, carvacrol and thymol show potent antiprotozoal activity without cytotoxicity. Planta Med. 2006, 72. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Zavareh, S.H.; Zangeneh, M. Antiparasitic activity of carvacrol obtained from Thymus caramanicus Jalas. Planta Med. 2008, 74. [Google Scholar] [CrossRef] [PubMed]
- Escobar, P.; Leal, S.M.; Herrera, L.V.; Martinez, J.R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem. Inst. Oswaldo Cruz 2010, 105, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-de-Melo, J.; Aparecida-Bitencourt, T.; Fachin, A.L.; Oliveira-Cruz, E.M.; Ramos-de-Jesus, H.C.; Barreto-Alves, P.; Arrigoni-Blank, M.D.F.; de Castro-Franca, S.; Oiveira-Beleboni, R.; Miranda-Fernandes, R.P.; et al. Antidermatophytic and antileishmanial activities of essential oils from Lippia gracilis Schauer genotypes. Acta Trop. 2013, 128, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Force, M.; Sparks, W.S.; Ronzio, R.A. Inhibition of enteric parasites by emulsified oil of Oregano in vivo. Phytother. Res. 2000, 14, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Elgemal, M.H.A.; Elewa, N.H.; Elkhisy, E.A.M.; Duddeck, H. 13C-NMR chemical shifts and carbon-proton coupling constants of some furocoumarins and furochromones. Phytochemistry 1979, 18, 139–143. [Google Scholar] [CrossRef]
- Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins: Plants, structure, properties. Chem. Nat. Comp. 1998, 34, 202–264. [Google Scholar] [CrossRef]
- Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins: Plants, structure, properties. Chem. Nat. Comp. 1998, 34, 345–409. [Google Scholar] [CrossRef]
- Friebolin, H. Basic One- and Two-Dimensional NMR Spectroscopy,, 2nd ed.; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1993. [Google Scholar]
- Kuffner, F.; Nikiforov, A.; Schulz, G. Über das Rutolid. Monatsh. Chem. 1973, 104, 911–915. [Google Scholar] [CrossRef]
- Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins: Plants, structure, properties. Chem. Nat. Comp. 1998, 34, 517–548. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Wang, J.J.; Iou, S.C.; Chang, H.C.; Chen, Y.P.; Kuo, Y.H.; Chang, Y.L.; Teng, C.M. Cytotoxic and antiplatelet aggregation principles of Ruta graveolens. J. Chin. Chem. Soc. 2003, 50, 171–178. [Google Scholar]
- Yang, Q.Y.; Tian, X.Y.; Fang, W.S. Bioactive coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007, 9, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.M.; Eble, J.N.; Starkovsky, N.A. Chalepensin, chalepin, and chalepin acetate, three novel furocoumarins from Ruta chalepensis. Lloydia 1967, 30, 73–77. [Google Scholar]
- Ezmirly, S.T.; Wilson, S.R. Saudi Arabian medicinal plants I: Ruta chalepensis. J. Chem. Soc. Pak. 1980, 2, 55–57. [Google Scholar]
- El-Beih, F.K.; El-Tawil, B.A.H.; Baghlaf, A.O. Constituents of local plants. Part 12. Coumarin and chalepensin, further constituents of Ruta chalepensis L. J. Chin. Chem. Soc. 1981, 28, 237–238. [Google Scholar]
- Ulubelen, A.; Güner, H. Isolation of dehydromoskachan C from Ruta chalepensis var. latifolia. J. Nat. Prod. 1988, 51, 1012–1013. [Google Scholar] [CrossRef]
- Ulubelen, A.; Güner, H.; Çetindağ, M. Alkaloids and coumarins from the roots of Ruta chalepensis var. latifolia. Planta Med. 1988, 54, 551–552. [Google Scholar] [CrossRef]
- El-Sayed, K.; Al-Said, M.S.; El-Feraly, F.S.; Ross, S.A. New quinolone alkaloids from Ruta chalepensis. J. Nat. Prod. 2000, 63, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Afifi-Yazar, F.U.; Shahadeh, M. Antiplatelet activity of Ruta chalepensis L. (Rutaceae) grown in Jordan. Planta Med. 2006, 72. [Google Scholar] [CrossRef]
- Macias, M.L.; Rojas, I.S.; Mata, R.; Lotina-Hennsen, B. Effect of selected coumarins on spinach chloroplast photosynthesis. J. Agric. Food Chem. 1999, 47, 2137–2140. [Google Scholar] [CrossRef] [PubMed]
- Anaya, A.L.; Macías-Rubalcava, M.; Cruz-Ortega, R.; García-Santana, C.; Sánchez-Monterrubio, P.N.; Hernández-Bautista, B.E.; Mata, R. Allelochemicals from Stauranthus perforatus, a Rutaceous tree of the Yucatan Peninsula, Mexico. Phythochemistry 2005, 66, 487–494. [Google Scholar] [CrossRef]
- Nebo, L.; Varela, R.M.; Molinillo, J.M.G.; Sampaio, O.M.; Severino, V.G.P.; Cazal, C.M.; Fernandes, M.F.D.G.; Fernandes, J.B.; Macías, F.A. Phytotoxicity of alkaloids, coumarins and flavonoids isolated from 11 species belonging to the Rutaceae and Meliaceae families. Phytochem. Lett. 2014, 8, 226–232. [Google Scholar] [CrossRef]
- Kong, Y.C.; Lau, C.P.; Wat, K.H.; Ng, K.H.; But, P.P.H.; Cheng, K.F.; Waterman, P.G. Antifertility principle of Ruta graveolens. Planta Med. 1989, 55, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Chaya, N.; Terauchi, K.; Yamagata, Y.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 14. coumarins and acridone alkaloids from Boenninghausenia japonica Nakai. Biol. Pharm. Bull. 2004, 27, 1312–1316. [Google Scholar]
- Ueng, Y.F.; Chen, C.C.; Chung, Y.T.; Liu, T.Y.; Chang, Y.P.; Lo, W.S.; Murayama, N.; Yamazaki, H.; Souček, P.; Chau, G.Y.; et al. Mechanism-based inhibition of cytochrome P450 (CYP)2A6 by chalepensin in recombinant systems, in human liver microsomes and in mice in vivo. Br. J. Pharmacol. 2011, 163, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.S.; Lim, Y.P.; Chen, C.C.; Hsu, C.C.; Souček, P.; Yun, C.H.; Xie, W.; Ueng, Y.F. A dual function of the furanocoumarin chalepensin in inhibiting Cyp2a and inducing Cyp2b in mice: The protein stabilization and receptor-mediated activation. Arch. Toxicol. 2012, 86, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Ueng, Y.F.; Chen, C.C.; Yamazaki, H.; Kiyotani, K.; Chang, Y.P.; Lo, W.S.; Li, D.T.; Tsai, P.L. Mechanism based inhibition of CYP1A1 and CYP3A4 by the furanocoumarin chalepensin. Drug Metab. Pharmacokinet. 2013, 28, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.C.; Mafezoli, J.; Pupo, M.T.; Fernandes, J.B.; da Silva, M.F.G.F.; de Albuquerque, S.; Oliva, G.; Pavão, F. Strategies for the isolation and identification of trypanocidal compounds from the Rutales. Pure Appl. Chem. 2001, 73, 617–622. [Google Scholar] [CrossRef]
- Pavão, F.; Castilho, M.S.; Pupo, M.T.; Dias, R.L.A.; Correa, A.G.; Fernandes, J.B.; da Silva, M.F.G.F.; Mafezoli, J.; Vieira, P.C.; Oliva, G. Structure of Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase complexed with chalepin, a natural product inhibitor, at 1.95 Å resolution. FEBS Lett. 2002, 520, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Said-Fernández, S.; Vargas-Villarreal, J.; Castro-Garza, J.; Mata-Cárdenas, B.D.; Navarro-Marmolejo, L.; Lozano-Garza, G.; Martínez-Rodríguez, H. PEHPS medium: An alternative for axenic cultivation of Entamoeba histolytica and E. invadens. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Mata-Cárdenas, B.D.; Vargas-Villarreal, J.; González-Salazar, F.; Palacios-Corona, R.; Said-Fernández, S. A new vial microassay to screen antiprotozoal drugs. Pharamacologyonline 2008, 1, 529–537. [Google Scholar]
- González-Salazar, F.; Mata-Cárdenas, B.D.; Vargas-Villarreal, J. Sensibilidad de trofozoitos de Entamoeba histolytica a ivermectina. Medicina (Buenos Aires) 2009, 69, 318–320. [Google Scholar]
- Chen, T.; Chen, L.; Li, H.; Chen, Y.; Guo, H.; Shu, Y.; Chen, Z.; Cai, C.; Guo, L.; Zhang, X.; et al. Design and in vitro evaluation of a novel poly(methacrylicacid)/metronidazole antibacterial nanogel as an oral dosage form. Colloids Surf. B 2014, 118, 65–71. [Google Scholar] [CrossRef]
- Sarker, M.M.A.; Rizwan, F.; Haque, R.; Siddique, A.; Parveen, S.; Islam, S. In vitro sensitivity of different brands of antiamoebic drugs (metronidazole tablets) against clinical isolates of Entamoeba histolytica in Bangladesh. J. Biol. Sci. 2008, 8, 925–929. [Google Scholar] [CrossRef]
- Miljkovic, V.; Arsic, B.; Bojanic, Z.; Nikolic, G.; Nikolic, L.J.; Kalicanin, B.; Savic, V. Interactions of metronidazole with other medicines: A brief review. Pharmazie 2014, 69, 571–577. [Google Scholar] [PubMed]
- Liu, C.T.; Tomsho, J.W.; Benkovic, S.J. The unique chemistry of benzoxaboroles: Current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014, 22, 4462–4473. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Licea, R.; Mata-Cárdenas, B.D.; Vargas-Villarreal, J.; Bazaldúa-Rodríguez, A.F.; Kavimngeles-Hernández, I.; Garza-González, J.N.; Hernández-García, M.E. Antiprotozoal Activity against Entamoeba histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia graveolens and Ruta chalepensis. Molecules 2014, 19, 21044-21065. https://doi.org/10.3390/molecules191221044
Quintanilla-Licea R, Mata-Cárdenas BD, Vargas-Villarreal J, Bazaldúa-Rodríguez AF, Kavimngeles-Hernández I, Garza-González JN, Hernández-García ME. Antiprotozoal Activity against Entamoeba histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia graveolens and Ruta chalepensis. Molecules. 2014; 19(12):21044-21065. https://doi.org/10.3390/molecules191221044
Chicago/Turabian StyleQuintanilla-Licea, Ramiro, Benito David Mata-Cárdenas, Javier Vargas-Villarreal, Aldo Fabio Bazaldúa-Rodríguez, Isvar Kavimngeles-Hernández, Jesús Norberto Garza-González, and Magda Elizabeth Hernández-García. 2014. "Antiprotozoal Activity against Entamoeba histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia graveolens and Ruta chalepensis" Molecules 19, no. 12: 21044-21065. https://doi.org/10.3390/molecules191221044
APA StyleQuintanilla-Licea, R., Mata-Cárdenas, B. D., Vargas-Villarreal, J., Bazaldúa-Rodríguez, A. F., Kavimngeles-Hernández, I., Garza-González, J. N., & Hernández-García, M. E. (2014). Antiprotozoal Activity against Entamoeba histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia graveolens and Ruta chalepensis. Molecules, 19(12), 21044-21065. https://doi.org/10.3390/molecules191221044