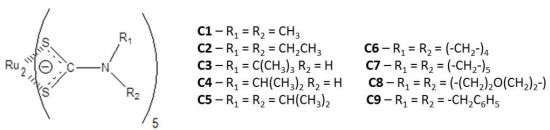
2.1. Chemistry
All dithiocarbamate ligands
L1–
L9 were synthesized in good yields (63%–93%) as described elsewhere [
42,
50] through the usual procedure starting from the corresponding substituted amines and carbon disulfide (CS
2); all ligands were obtained as pure products and have been well-characterized by the usual techniques (IR, ESI-MS,
1H and
13C-NMR spectra and elemental analysis). The coordination reaction between ruthenium (III) trichloride and the nine recently prepared dithiocarbamate ligands (
L1–
L9) (1:3 mol/mol, in ethanol) afforded nine pentakis(dithiocarbamate)diruthenium complexes
C1–
C9 (
C1:
N,
N-dimethyl
-;
C2:
N,
N-diethyl-;
C3:
N-mono-
tert-butyl-;
C4:
N-mono-(
iso-propyl)-;
C5:
N-di-(
iso-propyl)-;
C6:
N-pirrolidinyl-;
C7:
N-piperidinyl-;
C8:
N-morpholinyl- and
C9:
N,
N-dibenzyl-dithiocarbamates) in 65%–79% yield. The complete chemical characterization was made by several physicochemical techniques (measurement of magnetic susceptibility (μeff), electron paramagnetic resonance (EPR) spectra, conductivity, cyclic voltammetry) and spectrometric methods (IR, ESI-MS,
1H and
13C-NMR spectra) [
50,
59,
60,
61,
62]. All compounds were obtained in high purity level (TLC, HPLC and ESI(+)-MS) and the analysis of the results obtained by ESI-(+)-MS spectrometry suggested the general formula of [Ru
2(
Ln)
5] for all diruthenium pentadithiocarbamate complexes. In fact, the ESI-MS (positive and negative modes) indicated that the compounds are dinuclear neutral species, besides no chloride as counteranion was detected. If the chloride would be present, we should have seen an expected characteristic peak for chloride (
37Cl/
35Cl with a 1:3 ratio). The multiple fragmentation pattern observed in the ESI-MS spectra corroborates the obtaining of unsymmetrical dinuclear ruthenium complexes in which the dithiocarbamate moiety plays function as bridging ligand, since the presence of fragments that only can be formed through non-equivalent types of ruthenium-sulfur bonds is noticed, and if the compounds would be symmetrical (as in a mononuclear ruthenium complex or in a kind of oligomeric derivative) the fragmentation pattern would be much less complicated; this discussion will be reported further. There is a recent study [
63] about the UV-MALDI mass spectrometry investigation of well-known dithiocarbamate complexes (zinc, iron and manganese derivatives) that corroborates our structural proposal, since in this case symmetrical species (monomeric, dimeric, or tetrameric) were obtained and the corresponding mass spectra are not as complicated, as expected, compared to those in this present study. In addition, the analysis of magnetic susceptibility of the studied compounds showed values consistent with the proposed Ru(II)/Ru(III) system.
The proposal of a dinuclear Ru-Ru system is also corroborated by the infrared bands of ν(Ru-Ru) ≈ 205 cm
−1) and ν(S-Ru-S) ≈ 280 cm
−1). The former band associated with ν(Ru-Ru) should be IR-inactive due to the expected high symmetry of the system, nevertheless the observation of this band as cited elsewhere [
60] may indicate a distorted octahedral geometry around the diruthenium center. The electrochemical investigation by cyclic voltammetry also indicates that the complexes contain a neutral Ru(II)/Ru(III) system. Analysing the EPR results (
Figure 2) for the prepared dithiocarbamate complexes, typical EPR signalw of ruthenium(III) ions in the low spin 4d
5 configuration can be seen, as shown in
Figure 2 (for complexes
C2 (
Figure 2a) and
C4 (
Figure 2b)); the spike at 3,400 G (
g ≈ 2.00) in
Figure 2b is probably due to an organic decomposition product. It can be suggested that the obtained ruthenium compounds correspond to Ru(II)/Ru(III) mixed-valent states complexes, similar to a species already described in the literature, [Ru
2(acac)
4(μ-Q)]
+, where acac = 2,4-pentanedionate, and Q is a quinonoid group, with two equivalent π-conjugated α-diimine chelate sites, and one
p-quinone function [
64]. Those species can have different oxidation states accessible due to an intramolecular electron transfer, forming [Ru
III(μ-Q
2−)Ru
II] or [Ru
II(μ-Q
•−)Ru
II]. In the case of the dithiocarbamate ligands studied, formation of a radical species is also possible, giving rise to [Ru
IIL
2(μ-L)Ru
IIIL
2] or [Ru
IIL
2(μ-L
•)Ru
IIL
2] complexes.
Figure 2.
EPR spectra registered in solid state, at 77K, for complexes (a) C2 (α-pentakis(N,N-diethyldithiocarbamate)diruthenium [Ru2(S2CN(CH2CH3)2)5]) (b) C4 (α-pentakis(N-isopropyldithiocarbamate)diruthenium), [Ru2{S2CN(CH(CH3)2)(H)}5].
Figure 2.
EPR spectra registered in solid state, at 77K, for complexes (a) C2 (α-pentakis(N,N-diethyldithiocarbamate)diruthenium [Ru2(S2CN(CH2CH3)2)5]) (b) C4 (α-pentakis(N-isopropyldithiocarbamate)diruthenium), [Ru2{S2CN(CH(CH3)2)(H)}5].
2.2. Antifungal Assays
This study is one of the first approaches to the investigation of the potential usefulness of organoruthenium complexes as antifungal agents that could be used in IFIs. Activities have been determined against a limited number of isolates fungi, but they are representative of important filamentous fungal pathogens in humans.
The antifungal tests were carried out for all ruthenium complexes
C1–
C9 and the corresponding free dithiocarbamate ligands
L1–
L9, against six species (a total of thirteen different strains) of
Candida spp. (
Table 1), the most common fungal pathogen in IFIs [
6,
7]. In 96% of the cases the ruthenium complexes were more active than the corresponding free ligands (by 3-45 fold). It is important to notice that ruthenium trichloride (control test) did not show any significant antifungal activity for any of the studied microorganisms (>512 μg mL
−1/196 × 10
−5 mol L
−1). The most susceptible species were
C. albicans ATCC (
Table 1) and the clinical isolates 119CL and 01CL. Considering these three species the MIC decreasing activity order is:
C7 >
C6 >
C2 >
C1 >
C5 >
C9 >
C8 >
C3 >
C4. The most potent complexes
C7 and
C6 showed very low MIC values (0.40 and 0.43 × 10
−5 mol L
−1, respectively) that are comparable to the FLU result (0.33 × 10
−5 mol L
−1). Then, in a decreasing bioactivity order the complexes:
C2 >
C1 >
C5 >
C9 and
C8 are the most active ones (0.85, 1.0, 2.9, 4.1 and 6.3 × 10
−5 mol L
−1, respectively) and even the last and less potent complexes—
C3 and
C4 ‒ are still quite active (6.8 and 7.33 × 10
−5 mol L
−1, respectively). The clinical isolate
C. albicans 01CL was also very susceptible to the complexes in a similar decreasing (
C7 >
C2 ≥ C6 >
C1 >
C9 >
C5 >
C4 >
C8 >
C3) and with close MIC values, although a little higher (1.6–13.6 × 10
−5 mol L
−1) when compared to
Candida ATCC. These data reveal a significant relevancy of these ruthenium complexes as possible novel antifungal agent against IFIs, commonly related to candidiasis, and although AMB could be used, this drug is highly toxic, with low stability and fungal resistance to it has been observed [
9,
10,
11]. It can be noticed that the similar alkyl chain substituted pyrrolidinyl, ethyl, piperidinyl and the methyl derivatives
C7,
C2,
C6 and
C1 show the higher activity, while the mono-
N-alkyl analogues,
C4 (mono-
N-isopropyl) and
C3 (mono-
N-isopropyl), and the morpholine derivative (
C8) are the less active ones.
Among the others studied
Candida spp. (
C. krusei, C. parapsilosis, C. tropicalis and C. glabrata) (
Table 1), all studied complexes showed also good MIC results: 1.70–7.3, 0.8–4.1, 0.8–7.3 and 4.0–29.3 × 10
−5 mol L
−1, respectively and once more the ruthenium complexes were always more active than the corresponding free ligands (by 2–45 fold). Besides,
C. krusei and
C. parapsilosis are the most susceptible species and
C. glabrata is the less susceptible one (
Table 1). The most potent complexes against
C. parapsilosis are
C7,
C2 and
C6 (MIC: 0.80, 0.85 and 0.86 × 10
−5 mol L
−1) and it is observed that these three complexes are almost as potent as FLU (0.33 × 10
−5 mol.L
−1), generally the first drug option for drug-resistant fungal disease. Again, similar decreasing activity orders for the studied ruthenium complexes can be pointed out for these three species of
Candida:
C. krusei,
C. parapsilosis,
C. tropicalis:
C7 >
C2 ≥ C6 >
C1 ≥ C9 >
C5 >
C8 >
C4 >
C3;
C7 ≥ C2 ≥ C6 >
C1 >
C5 ≥ C8 >
C9 >
C3 >
C4;
C7 ≥ C2 ≥ C6 >
C1 >
C3 >
C9 > C5 >
C8 >
C4, respectively. In the case of
C. glabrata the activity order for the complexes is completely different:
C1 ≥ C2 >
C9 >
C5 ≈ C7 ≈ C8 ≈ C6 ≈ C4 ≈ C3.
Table 1.
In vitro antifungal activity of pentakis-dithiocarbamate diruthenium complexes and the corresponding free ligands (MIC, μg mL−1/10−5mol L−1) against different species of Candida by microdilution method.
Table 1.
In vitro antifungal activity of pentakis-dithiocarbamate diruthenium complexes and the corresponding free ligands (MIC, μg mL−1/10−5mol L−1) against different species of Candida by microdilution method.
| Compounds | Candida albicans | C. krusei | C. parapsilosis | C. tropicalis | C. glabrata |
|---|
| ATCC | 119CL | 01CL |
|---|
| L1 | 16 (11) | 16 (11) | 16 (11) | 16 (11.2) | 128 (89.4) | 64 (44.7) | - |
| C1 | 8.0 (1.0) | 16 (2.0) | 16 (2.0) | 16 (2.0) | 16 (2.0) | 16 (2.0) | 32 (4.0) |
| L2 | 16 (9.3) | 16 (9.3) | 16 (9.3) | 16 (9.3) | 64 (37) | 64 (37) | - |
| C2 | 8.0 (0.85) | 16 (1.7) | 16 (1.70) | 16 (1.70) | 8 (0.9) | 8 (0.9) | 64 (6.8) |
| L3 | 64 (37) | 32 (19) | 32 (18.7) | 128 (74.7) | 32 (18.7) | 128 (74.7) | - |
| C3 | 64 (6.8) | 128 (13.6) | 128 (13.6) | 64 (6.8) | 64 (6.8) | 32 (3.4) | 256 (27.1) |
| L4 | 32 (21) | 16 (10) | 16 (10) | 16 (10) | 32 (21) | 64 (42) | - |
| C4 | 64 (7.3) | 64 (7.3) | 64 (7.3) | 64 (7.3) | 64 (7.3) | 64 (7.3) | 256 (29.3) |
| L5 | 128 (64) | 32 (16) | 32 (16) | 64 (32) | 64 (32) | 128 (64) | - |
| C5 | 32 (3.0) | 64 (5.9) | 64 (5.9) | 32 (3.0) | 32 (3.0) | 64 (5.9) | 256 (23.6) |
| L6 | 2.0 (1.2) | 16 (9.5) | 16 (9.45) | 8 (4.7) | 16 (9.5) | 16 (9.5) | - |
| C6 | 4.0 (0.43) | 16 (1.7) | 16 (1.71) | 16 (1.7) | 8.0 (0.86) | 8.0 (0.86) | 256 (27.4) |
| L7 | 8.0 (4.4) | 16 (8.8) | 16 (8.8) | 8.0 (4.4) | 32 (18) | 32 (18) | - |
| C7 | 4.0 (0.40) | 16 (1.6) | 16 (1.6) | 16 (1.6) | 8.0 (0.8) | 8.0 (0.8) | 256 (25.4) |
| L8 | 128 (69.1) | 128 (69.1) | 128 (69.1) | 128 (69.1) | 128 (69.1) | 128 (69.1) | - |
| C8 | 64 (6.3) | 64 (6.3) | 128 (12.6) | 64 (6.4) | 32 (3.2) | 64 (6.4) | 256 (25.6) |
| L9 | 8.0 (3.0) | 128 (47.9) | 64 (24) | 8.0 (3.0) | 32 (12) | 32 (12) | - |
| C9 | 64 (4.1) | 64 (4.1) | 64 (4.1) | 32 (2.1) | 64 (4.1) | 64 (4.1) | 256 (16.4) |
| RuCl3 | >512 (196) | >512 (196) | >512 (196) | >512 (196) | >512 (196) | >512 (196) | >512 (196) |
| AMB | 0.50 (0.05) | 1.00 (0.11) | 0.50 (0.05) | 0.25 (0.03) | 0.50 (0.05) | 1.00 (0.11) | 0.50 (0.05) |
| FLU | 1.00 (0.33) | 1.00 (0.33) | 32.0 (10.4) | 1.00 (0.33) | 1.00 (0.33) | 32.0 (10.4) | 1.00 (0.33) |
Considering the antifungal activity tests against
C. dubliniensis (
Table 2), the CD29 and the CD22 clinical isolates were the less susceptible species, but even for CD22 and the complex
C1, and for
C27 and
C2 the obtained MIC values were comparable, and even lower (8.0 and 6.8 × 10
−5 mol L
−1, respectively) than the obtained ones with FLU (10.4 × 10
−5 mol L
−1). All complexes and analogous free ligands were also tested against the
C. dubliniensis CD28 clinical isolate (
Table 3). All complexes and all free ligands were active (MIC: 1.7–16.1 10
−5 mol L
−1), except
L8, that showed very low activity (MIC 69.1 × 10
−5 mol L
−1). The decreasing activity order of the studied complexes was also similar to the previous ones:
C7 >
C2 = C6 >
C1 >
C5 >
C9 >
C8 >
C4 >
C3. Although the interesting results obtained, none of them showed lower MIC values than FLU, and as in all cases before, except for
C. glabrata, the preliminary structure-activity relationship analysis shows that the mono-
N-alkyl derivatives
C3 and
C4 are the less active compounds and the bis-
N,
N-dialkyldithiocarbamate complexes are the most active ones.
In the susceptibility tests of seven clinical isolates of
Paracoccidioides brasiliensis (
Table 4)
, it can be noticed that the obtained results of the different clinical isolates were quite similar (0.42–12.6 × 10
−5 mol L
−1), but the clinical isolate MG05 was the most susceptible species (0.42–1.70 × 10
−5 mol L
−1) and that the complexes were also more active than the corresponding free ligands (3–180 fold).
Table 2.
In vitro susceptibility of species of Candida dubliniensis clinical isolates for L1, C1, L2 and C2 complexes by microdilution methods—MIC: μg mL−1 (10−5mol L−1).
Table 2.
In vitro susceptibility of species of Candida dubliniensis clinical isolates for L1, C1, L2 and C2 complexes by microdilution methods—MIC: μg mL−1 (10−5mol L−1).
| Compounds | Candida dubliniensisclinical isolates |
|---|
| CD22 | CD23 | CD25 | CD27 | CD29 |
|---|
| L1 | 8.0 (5.6) | 4 (2.8) | 8 (5.6) | 4 (2.8) | 8 (5.6) |
| C1 | 64 (8.0) | 8.0 (1.0) | 8 (1.0) | 8 (1.00) | 32 (4.00) |
| L2 | 8.0 (4.6) | 4.0 (2.4) | 8.0 (4.8) | 8.0 (4.8) | 8.0 (4.8) |
| C2 | 64 (6.8) | 64 (6.8) | 64 (6.8) | 32 (3.4) | 64 (6.8) |
| AMB | 0.50 (0.05) | 1.0 (0.10) | 0.50 (0.05) | 0.50 (0.05) | 1 (0.10) |
| RuCl3 | >512 (196) | >512 (196) | >512 (196) | >512 (196) | >512 (196) |
| FLU | 32.0 (10.2) | 1.00 (0.32) | 1.00 (0.32) | 32.0 (10.2) | 1.00 (0.32) |
Table 3.
In vitro susceptibility—MIC: μg mL−1 (10−5mol L−1) of Candida dubliniensis clinical isolate CD28 for pentakis-dithiocarbamate diruthenium complexes and free ligands by microdilution methods.
Table 3.
In vitro susceptibility—MIC: μg mL−1 (10−5mol L−1) of Candida dubliniensis clinical isolate CD28 for pentakis-dithiocarbamate diruthenium complexes and free ligands by microdilution methods.
| | L1 | C1 | L2 | C2 | L3 | C3 | L4 | C4 | L5 | C5 |
|---|
| CD28 | 8 | 16 | 8 | 16 | 16 | 64 | 16 | 64 | 32 | 32 |
| (5.6) | (2.0) | (4.7) | (1.7) | (9.3) | (7.8) | (10) | (7.3) | (16.1) | (3.0) |
| | L6 | C6 | L7 | C7 | L8 | C8 | L9 | C9 | AMB | FLU |
| CD28 | 16 | 16 | 16 | 16 | 128 | 64 | 64 | 64 | 0.50 | 1.00 |
| (9.5) | (1.7) | (8.7) | (1.6) | (69.1) | (6.3) | (24) | (4.1) | (0.11) | (0.33) |
Table 4.
In vitro susceptibility of clinical isolates of Paracoccidioides brasiliensis for pentakis-dithiocarbamate diruthenium complexes by microdilution methods—MIC: μg mL−1 (10−5 mol L−1).
Table 4.
In vitro susceptibility of clinical isolates of Paracoccidioides brasiliensis for pentakis-dithiocarbamate diruthenium complexes by microdilution methods—MIC: μg mL−1 (10−5 mol L−1).
| Compounds | Paracocidioides brasiliensis Clinical Isolates |
|---|
| MG05 | PB01 | PB18 | PB017 | 608 | B339 | MG04 |
|---|
| C1 | 16 (2.0) | 32 (4.0) | 16 (2.0) | 32 (4.0) | 32 (4.0) | 16 (2.0) | 16 (2.0) |
| C2 | 4.0 (0.42) | 8.0 (0.85) | 16 (1.7) | 8 (0.85) | 16 (1.7) | 4.0 (0.42) | 8.0 (0.85) |
| C3 | 64 (6.8) | 64 (6.8) | 64 (6.8) | 64 (6.8) | 64 (6.8) | 64 (6.8) | 64 (6.8) |
| C4 | 64 (7.3) | 64 (7.3) | 64 (7.3) | 64 (7.3) | 64 (7.3) | 64 (7.3) | 64 (7.3) |
| C5 | 64 (5.9) | 64 (5.9) | 64 (5.9) | 64 (5.9) | - | - | - a |
| C6 | 16 (1.7) | 16 (1.7) | 16 (1.7) | 16 (1.7) | - | - | - a |
| C7 | 16 (1.6) | 16 (1.6) | 16 (1.6) | 16 (1.6) | - | - | - a |
| C8 | 128 (12.6) | 64 (6.3) | 128 (12.6) | 128 (12.6) | 128 (12.6) | 128 (12.6) | 128 (12.6) |
| C9 | 128 (8.18) | 64 (4.1) | 128 (8.2) | 128 (8.2) | 128 (8.2) | 128 (8.2) | 128 (8.2) |
| RuCl3 | >512 (196) | >512 (196) | >512 (196) | >512 (196) | >512 (196) | >512 (196) | >512 (196) |
| AMB | 0.50 (0.05) | 1.00 (0.11) | 0.50 (0.05) | 0.25 (0.03) | 0.50 (0.05) | 1.00 (0.11) | 0.50 (0.05) |
| FLU | 1.00 (0.33) | 1.00 (0.33) | 32.0 (10.4) | 1.00 (0.33) | 1.00 (0.33) | 32.0 (10.4) | 1.00 (0.33) |
The complexes C2, C7, C6 and C1 showed the highest activities (respective MIC values: 0.42, 1.6, 1.7 and 2.0 × 10−5 mol L−1) followed by compounds C5, C3, C4, C9 and C8 (5.9, 6.8, 7.3, 8.18 and 12.6 × 10−5 mol L−1). The four former complexes really showed antifungal activity similar to FLU (0.33–10.4 × 10−5 mol L−1). These results suggest that the similar derivatives with ethyl (C2), tetramethylene and pentamethylene (C6 and C7), and methyl (C1) moieties are the most active compounds.
The susceptibility tests of
Cryptococcus neoformans (
Table 5) were also performed and as observed before, the free ligands were less active than the ruthenium complexes (3–11 fold), but when the studied complexes are compared to each other, the results were different than the other ones described above. The
N,
N-dibenzyldithiocarbamate diruthenium complex
C9 was the most active agent (MIC: 1.02 × 10
−5 mol L
−1) followed by
C5,
C7,
C2,
C6 and
C1 with very similar MIC values (1.48–1.99 × 10
−5 mol L
−1) and by
C8,
C4 and
C3 (3.16, 3.66 and 6.8 × 10
−5 mol L
−1). In fact, in this case a preliminary structure-activity relationship shows a clear correlation between the steric hindrance of the
N,
N-dialkylsubstituents of the complexes and the antifungal activity:
C9 (
N,
N-dibenzyl) >
C5 (
N,
N-diisopropyl) ≥
C7 (piperidinyl) ≥
C2 (diethyl) ≥
C6 (pyrrolidinyl) >
C1 (dimethyl). The mono-
N-alkyl complexes –
C3 (mono-
tert-butyl) and
C4 (mono-isopropyl) ‒ and the morpholine derivative
C8, with an oxygen atom in the ring of the substituent, were the less active compounds. It is remarkable that
C9 showed itself almost as potent as FLU (0.65 × 10
−5 mol L
−1).
Table 5.
In vitro susceptibility of clinical isolates of Cryptococcus neoformans for pentakis-dithiocarbamate diruthenium complexes by broth microdilution methods—MIC: μg mL−1 (10−5 mol L−1).
Table 5.
In vitro susceptibility of clinical isolates of Cryptococcus neoformans for pentakis-dithiocarbamate diruthenium complexes by broth microdilution methods—MIC: μg mL−1 (10−5 mol L−1).
| Compounds | Cryptococcus neoformans | Compounds | Cryptococcus neoformans |
|---|
| L1 | 16.0 (11.2) | L6 | 16.0 (9.45) |
| C1 | 16.0 (1.99) | C6 | 16.0 (1.71) |
| L2 | 16.0 (9.34) | L7 | 16.0 (8.73) |
| C2 | 16.0 (1.70) | C7 | 16.0 (1.59) |
| L3 | 16.0 (9.34) | L8 | 64.0 (34.5) |
| C3 | 64.0 (6.80) | C8 | 32.0 (3.16) |
| L4 | 16.0 (10.2) | L9 | 128 (47.9) |
| C4 | 32.0 (3.66) | C9 | 16.0 (1.02) |
| L5 | 32.0 (16.0) | RuCl3 | >512 (196) |
| C5 | 16.0 (1.48) | FLU | 2.0 (0.65) |
Only the acyclic derivatives
C1–
C5 could be tested on
Sporotrix schenckii (
Table 6) since the tests with the other complexes could not be performed due to cell growth problems. The
N,
N-dialkyldithiocarbamate ruthenium complexes
C2–
C1 showed higher activity (MIC:
C2-0.85 and
C1-1.0 × 10
−5 mol L
−1) followed by the mono-
N-alkyl analogues
C4 and
C3 (MIC:
C4-14.8 and
C3-27.1 × 10
−5 mol L
−1); the
N,
N-diisopropyl complex
C5 did not show interesting antifungal activity (MIC > 14.8 × 10
−5 mol L
−1).
C1 and
C2 indeed showed MIC values that are closely comparable with those obtained with FLU (0.65 × 10
−5 mol L
−1).