Mesoporous Silicas with Tunable Morphology for the Immobilization of Laccase
Abstract
:1. Introduction
2. Results and Discussion
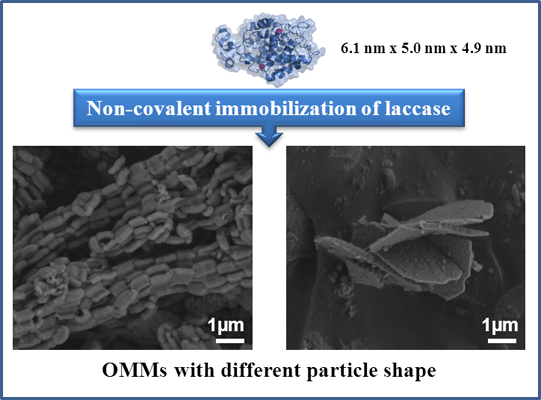
2.1. Characterization of Supports




2.2. Immobilization of Laccase and Specific Activity



| Support | pH immob. a | tc (h) b | % Immob. c | Enzyme loading (mg/g) d | Specific activity (U/mg) e |
|---|---|---|---|---|---|
| SBA-15-L | 3.5 | 3 | 62.56 | 31.28 | 0.134 |
| SBA-15-S | 3.5 | 3 | 76.82 | 38.41 | 0.159 |
| MS-3030 | 3.5 | 3 | 31.76 | 15.88 | 0.014 |
| MS-3030-N | 5.5 | 3 | 99.28 | 49.64 | 0.981 |
2.3. Leaching Test of Immobilized Laccase


3. Experimental
3.1. Synthesis of the Supports
3.2. Characterization Techniques
3.3. Protein Determination and Activity Assay
3.4. Immobilization of Laccase on Mesoporous Silicates
3.5. Immobilized Laccase Leaching Tests
3.6. SDS-PAGE Electrophoresis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartmann, M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Hudson, S.; Magner, E.; Cooney, J.; Hodnett, B.K. Methodology for the immobilization of enzymes onto mesoporous materials. J. Phys. Chem. B 2005, 109, 19496–19506. [Google Scholar] [CrossRef]
- Serra, E.; Mayoral, A.; Sakamoto, Y.; Blanco, R.M.; Diaz, I. Immobilization of lipase in ordered mesoporous materials: Effect of textural and structural parameters. Microporous Mesoporous Mater. 2008, 114, 201–213. [Google Scholar] [CrossRef]
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas—Benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartmann, M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef]
- Chong, A.S.M.; Zhao, X.S. Functionalization of SBA-15 with APTES and characterization of functionalized materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar] [CrossRef]
- Blanco, R.M.; Terreros, P.; Fernandez-Perez, M.; Otero, C.; Diaz-Gonzalez, G. Functionalization of mesoporous silica for lipase immobilization—Characterization of the support and the catalysts. J. Mol. Catal. B: Enzym. 2004, 30, 83–93. [Google Scholar] [CrossRef]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent advances in functionalization of mesoporous silica. J. Nanosci. Nanotechnol. 2005, 5, 347–371. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascon, V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef]
- Athens, G.L.; Shayib, R.M.; Chmelka, B.F. Functionalization of mesostructured inorganic-organic and porous inorganic materials. Curr. Opin. Colloid Interface Sci. 2009, 14, 281–292. [Google Scholar] [CrossRef]
- Zhao, X.S.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32–39. [Google Scholar] [CrossRef]
- Tran, D.N.; Balkus, K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, M.M.; Gao, L.; Lin, N.; Lin, W.G.; Zhu, J.H. One-pot synthesis of a hierarchical PMO monolith with superior performance in enzyme immobilization. J. Mater. Chem. B 2013, 1, 1738–1748. [Google Scholar] [CrossRef]
- Leonowicz, A.; Cho, N.S.; Luterek, J.; Wilkolazka, A.; Wojtas-Wasilewska, M.; Matuszewska, A.; Hofrichter, M.; Wesenberg, D.; Rogalski, J. Fungal laccase: Properties and activity on lignin. J. Basic Microbiol. 2001, 41, 185–227. [Google Scholar] [CrossRef]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochem. Rev. 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Kunamneni, A.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Laccases and their applications: A patent review. Recent Pat. Biotechnol. 2008, 2, 10–24. [Google Scholar] [CrossRef]
- Brijwani, K.; Rigdon, A.; Vadlani, P.V. Fungal laccases: Production, function, and applications in food processing. Enzym. Res. 2010, 2010, 149748. [Google Scholar]
- Minussi, R.C.; Pastore, G.M.; Duran, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Rodriguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, G.; Niu, J.; Wang, Y.; Hu, L. Laccase-catalyzed oxidation of organic pollutants in water. Prog. Chem. 2009, 21, 2028–2036. [Google Scholar]
- Osma, J.F.; Toca-Herrera, J.L.; Rodriguez-Couto, S. Uses of laccases in the food industry. Enzym. Res. 2010, 2010, 918761. [Google Scholar]
- Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Burton, S. Potential applications of laccase-mediated coupling and grafting reactions: A review. Enzym. Microb. Technol. 2011, 48, 195–208. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, M.; Sanroman, M.A.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System. Available online: http://www.pymol.org/ (accessed on 14 January 2014).
- Berka, R.M.; Schneider, P.; Golightly, E.J.; Brown, S.H.; Madden, M.; Brown, K.M.; Halkier, T.; Mondorf, K.; Xu, F. Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl. Environ. Microbiol. 1997, 63, 3151–3157. [Google Scholar]
- Fan, J.; Lei, J.; Wang, L.M.; Yu, C.Z.; Tu, B.; Zhao, D.Y. Rapid and high-capacity immobilization of enzymes based on mesoporous silicas with controlled morphologies. Chem. Commun. 2003, 2140–2141. [Google Scholar]
- Fernandez, O.; Diaz, I.; Torres, C.F.; Tobajas, M.; Tejedor, V.; Blanco, R.M. Hybrid composites octyl-silica-methacrylate agglomerates as enzyme supports. Appl. Catal. A 2013, 450, 204–210. [Google Scholar] [CrossRef]
- Weber, E.; Sirim, D.; Schreiber, T.; Thomas, B.; Pleiss, J.; Hunger, M.; Glaser, R.; Urlacher, V.B. Immobilization of P450 BM-3 monooxygenase on mesoporous molecular sieves with different pore diameter. J. Mol. Catal. B-Enzym. 2010, 64, 29–37. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Linton, P.; Alfredsson, V. Growth and Morphology of Mesoporous SBA-15 Particles. Chem. Mater. 2008, 20, 2878–2880. [Google Scholar] [CrossRef]
- Linton, P.; Rennie, A.R.; Zackrisson, M.; Alfredsson, V. In situ observation of the genesis of mesoporous silica SBA-15: Dynamics on length scales from 1 nm to 1 microm. Langmuir 2009, 25, 4685–4691. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid inorganic-organic mesoporous silicates—Nanoscopic reactors coming of age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Leonowicz, A.; Grzywnowicz, K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzym. Microb. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]
- Sanchez-Amat, A.; Solano, F. A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp shares catalytic capabilities of tyrosinases and laccases. Biochem. Biophys. Res. Commun. 1997, 240, 787–792. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gascón, V.; Díaz, I.; Márquez-Álvarez, C.; Blanco, R.M. Mesoporous Silicas with Tunable Morphology for the Immobilization of Laccase. Molecules 2014, 19, 7057-7071. https://doi.org/10.3390/molecules19067057
Gascón V, Díaz I, Márquez-Álvarez C, Blanco RM. Mesoporous Silicas with Tunable Morphology for the Immobilization of Laccase. Molecules. 2014; 19(6):7057-7071. https://doi.org/10.3390/molecules19067057
Chicago/Turabian StyleGascón, Victoria, Isabel Díaz, Carlos Márquez-Álvarez, and Rosa M. Blanco. 2014. "Mesoporous Silicas with Tunable Morphology for the Immobilization of Laccase" Molecules 19, no. 6: 7057-7071. https://doi.org/10.3390/molecules19067057
APA StyleGascón, V., Díaz, I., Márquez-Álvarez, C., & Blanco, R. M. (2014). Mesoporous Silicas with Tunable Morphology for the Immobilization of Laccase. Molecules, 19(6), 7057-7071. https://doi.org/10.3390/molecules19067057