Effect of Structure on the Interactions between Five Natural Antimicrobial Compounds and Phospholipids of Bacterial Cell Membrane on Model Monolayers
Abstract
:1. Introduction

2. Results and Discussion
2.1. Compression Isotherm and Compressibility Modulus of Monolayer
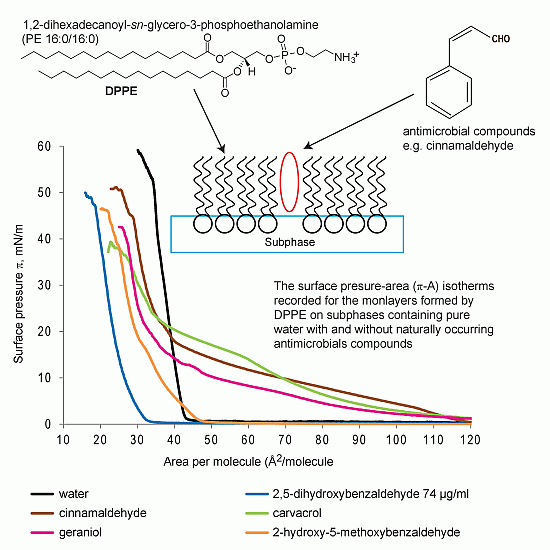
2.1.1. DPPE Monolayer

2.1.2. DPPG Monolayer

2.2. Cardiolipin Monolayer

2.3. Comparison of Compression Isotherms of the Three Phospholipid Monolayers
2.4. Surface Potential of the Monolayer
2.4.1. DPPE Monolayer

2.4.2. DPPG Monolayer

2.5. Cardiolipin Monolayer

2.6. Mechanistic Aspects
| Monolayer Parameter | Test Substance | |||||
|---|---|---|---|---|---|---|
| Water | Carvacrol | Cinnamal-dehyde | Geraniol | 2,5-Dihydroxy-benzaldehyde | 2-Hydroxy-5-methoxy-benzaldehyde | |
| DPPE | ||||||
| lift-off value (Å2/molecule) | 43 | 120 | 117 | 120 | 33 | 43 |
| collapse (mN/m)pressure | 58 | 38 | 51 | 43 | 50 | 47 |
| compressibility-surface pressure (Cs−1) | 240 | 50 | 95 | 88 | 108 | 100 |
| surface potential (mV) | 600 | 520 | 300 | 300 | 300 | 600 |
| surface dipole moment area (Å2/molecule) | 185 | 75 | 100 | 90 | 75 | |
| DPPG | ||||||
| lift-off value (Å2/molecule) | 90 | 200 | 200 | 200 | 90 | 125 |
| collapse (mN/m)pressure | 49 | 42 | 44 | 38 | 52 | 42 |
| compressibility-surface pressure (Cs−1) | 255 | 60 | 175 | 80 | 170 | 72 |
| surface potential (mV) | 450 | 180 | 75 | 25 | 230 | 460 |
| surface dipole moment area (Å2/molecule) | 125 | 400 | 250 | 180 | 270 | 250 |
| Cardiolipin | ||||||
| lift-off value (Å2/molecule) | 110 | 180 | 180 | 180 | 140 | 180 |
| collapse (mN/m)pressure | 52 | 39 | 43 | 32 | 52 | 44 |
| compressibility-surface pressure (Cs−1) | 145 | 85 | 120 | 90 | 130 | 115 |
| surface potential (mV) | 270 | 280 | 80 | −100 | 430 | 450 |
| surface dipole moment area (Å2/molecule) | 260 | 400 | 400 | 250 | 320 | 210 |
3. Experimental
3.1. Materials
3.2. Monolayer Measurements
3.3. Analysis of Isotherms
4. Conclusions
Abbreviations
| Cs−1 | isothermal compressibility of monolayer |
| Cs−1-π | compressibility-surface pressure of monolayer |
| DPPE | 1,2-dihexadecanoyl-sn-glycero-3-phosphoethylamine |
| DPPG | 1,2-dihexadecanoyl-sn-glycero-3-phospho-(1'-rac-glycerol) |
| LC | liquid-condensed |
| LE | liquid-expanded |
| LE-LC | two-dimensional phase transition |
| LPS | lipopolysaccharide |
| LTA | lipoteichoic acid |
| MIC | minimum inhibitory concentration |
| POPC | 1-palmitoyl-2-oleoylphosphatidylcholine |
| π | surface pressure of monolayer |
| πA | surface pressure molecular area of monolayer |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.E.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular binding of catechins to biomembranes: Relationship to biological activity. J. Agric. Food Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Lopes, J.L.S.; Nobre, T.M.; Siano, Á.; Humpola, V.; Bossolan, N.R.S.; Zaniquelli, M.E.D.; Tonarelli, G.; Beltramini, L.M. Disruption of Saccharomyces cerevisiae by Plantaricin 149 and investigation of its mechanism of action with biomembrane model systems. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2252–2258. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Maget-Dana, R. The monolayer technique: A potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim. Biophys. Acta 1999, 1462, 109–140. [Google Scholar]
- Gagos, M.; Arczewska, M. FTIR spectroscopic study of molecular organization of the antibiotic amphotericin B in aqueous solution and in DPPC lipid monolayers containing the sterols cholesterol and ergosterol. Eur. Biophys. J. 2012, 41, 663–673. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Janczyk, A. Probing the modes of antibacterial activity of chitosan. Effects of pH and molecular weight on chitosan interactions with membrane lipids in Langmuir films. Biomacromolecules 2011, 12, 4144–4152. [Google Scholar] [CrossRef]
- Pinheiro, M.; Giner-Casares, J.J.; Lúcio, M.; Caio, J.M.; Moiteiro, C.; Lima, J.L.F.C.; Reis, S.; Camacho, L. Interplay of mycolic acids, antimycobacterial compounds and pulmonary surfactant membrane: A biophysical approach to disease. Biochim. Biophys. Acta Biomembr. 2013, 1828, 896–905. [Google Scholar]
- Salay, L.C.; Ferreira, M.; Oliveira, O.N., Jr.; Nakaie, C.R.; Schreier, S. Headgroup specificity for the interaction of the antimicrobial peptide tritrpticin with phospholipid Langmuir monolayers. Colloids Surf. B 2012, 100, 95–102. [Google Scholar] [CrossRef]
- Feng, S.S. Interpretation of mechanochemical properties of lipid bilayer vesicles from the equation of state or pressure-area measurement of the monolayer at the air-water or oil-water interface. Langmuir 1999, 15, 998–1010. [Google Scholar] [CrossRef]
- Brockman, H. Lipid monolayers: Why use half a membrane to characterize protein-membrane interactions? Curr. Opin. Struct. Biol. 1999, 9, 438–443. [Google Scholar] [CrossRef]
- Marsh, D. Lateral pressure in membranes. Biochim. Biophys. Acta Rev. Biomembr. 1996, 1286, 183–223. [Google Scholar]
- Wydro, P.; Witkowska, K. The interactions between phosphatidylglycerol and phosphatidylethanolamines in model bacterial membranes. The effect of the acyl chain length and saturation. Colloids Surf. B 2009, 72, 32–39. [Google Scholar]
- Krajewska, B.; Kyziol, A.; Wydro, P. Chitosan as a subphase disturbant of membrane lipid monolayers. The effect of temperature at varying pH: II. DPPC and cholesterol. Colloids Surf. Physicochem. Eng. Asp. 2013, 434, 359–364. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Kyziol, A. Chitosan as a subphase disturbant of membrane lipid monolayers. The effect of temperature at varying pH: I. DPPG. Colloids Surf. Physicochem. Eng. Asp. 2013, 434, 349–358. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar]
- Friedman, M.; Buick, R.; Elliott, C.T. Antibacterial activities of naturally occurring compounds against antibiotic-resistant Bacillus cereus vegetative cells and spores, Escherichia coli, and Staphylococcus aureus. J. Food Prot. 2004, 67, 1774–1778. [Google Scholar]
- Wong, S.Y.Y.; Grant, I.R.; Friedman, M.; Elliott, C.T.; Situ, C. Antibacterial activities naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 2008, 74, 5986–5990. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Domains in bacterial membranes and the action of antimicrobial agents. Mol. BioSyst. 2009, 5, 580–587. [Google Scholar]
- Epand, R.M.; Epand, R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta 2009, 1788, 289–294. [Google Scholar]
- Cronan, J.E. Bacterial membrane lipids: Where do we stand? Annu. Rev. Microbiol. 2003, 57, 203–224. [Google Scholar]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef]
- Sirk, T.W.; Friedman, M.; Brown, E.F. Molecular binding of black tea theaflavins to biological membranes: Relationship to bioactivities. J. Agric. Food Chem. 2011, 59, 3780–3787. [Google Scholar] [CrossRef]
- Smaby, J.M.; Kulkarni, V.S.; Momsen, M.; Brown, R.E. The interfacial elastic packing interactions of galactosylceramides, sphingomyelins, and phosphatidylcholines. Biophys. J. 1996, 70, 868–877. [Google Scholar] [CrossRef]
- Dynarowicz-Latka, P.; Dhanabalan, A.; Oliveira, O.N., Jr. Modern physicochemical research on Langmuir monolayers. Adv. Colloid Interface Sci. 2001, 91, 221–293. [Google Scholar] [CrossRef]
- Bernsdorff, C.; Winter, R. Differential properties of the sterols cholesterol, ergosterol, b-sitosterol, trans-7-dehydrocholesterol, stigmasterol and lanosterol on DPPC bilayer order. J. Phys. Chem. B 2003, 107, 10658–10664. [Google Scholar] [CrossRef]
- Nes, I.F.; Eklund, T. The effect of parabens on DNA, RNA and protein synthesis in Escherichia coli and Bacillus subtilis. J. Appl. Bacteriol. 1983, 54, 237–242. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Friedman, M. Antibiotic activities of plant compounds against non-resistant and antibiotic-resistant foodborne human pathogens. ACS Symp. Ser. 2006, 931, 167–183. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeerdsma-van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural requirements for the antimicrobial activity of carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Giordani, C.; Molinari, A.; Toccacieli, L.; Calcabrini, A.; Stringaro, A.; Chistolini, P.; Arancia, G.; Diociaiuti, M. Interaction of tea tree oil with model and cellular membranes. J. Med. Chem. 2006, 49, 4581–4588. [Google Scholar] [CrossRef]
- Bergstrom, C.L.; Beales, P.A.; Lv, Y.; Vanderlick, T.K.; Groves, J.T. Cytochrome c causes pore formation in cardiolipin-containing membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 6269–6274. [Google Scholar]
- Lopes, S.C.; Neves, C.S.; Eaton, P.; Gameiro, P. Improved model systems for bacterial membranes from differing species: The importance of varying composition in PE/PG/cardiolipin ternary mixtures. Mol. Membr. Biol. 2012, 29, 207–217. [Google Scholar] [CrossRef]
- Jones, E.M.; Dubey, M.; Camp, P.J.; Vernon, B.C.; Biernat, J.; Mandelkow, E.; Majewski, J.; Chi, E.Y. Interaction of tau protein with model lipid membranes induces tau structural compaction and membrane disruption. Biochemistry 2012, 51, 2539–2550. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Hua, W.; Castada, H.; Allen, H.C. Reorganization and caging of DPPC, DPPE, DPPG, and DPPS monolayers caused by dimethylsulfoxide observed using brewster angle microscopy. Langmuir 2010, 26, 18902–18908. [Google Scholar] [CrossRef]
- Yi, Z.; Nagao, M.; Bossev, D.P. Effect of charged lidocaine on static and dynamic properties of model bio-membranes. Biophys. Chem. 2012, 160, 20–27. [Google Scholar] [CrossRef]
- Teruel, J.A.; Ortiz, A.; Aranda, F.J. Interactions of a bacterial trehalose lipid with phosphatidylglycerol membranes at low ionic strength. Chem. Phys. Lipids 2014, 181, 34–39. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nowotarska, S.W.; Nowotarski, K.J.; Friedman, M.; Situ, C. Effect of Structure on the Interactions between Five Natural Antimicrobial Compounds and Phospholipids of Bacterial Cell Membrane on Model Monolayers. Molecules 2014, 19, 7497-7515. https://doi.org/10.3390/molecules19067497
Nowotarska SW, Nowotarski KJ, Friedman M, Situ C. Effect of Structure on the Interactions between Five Natural Antimicrobial Compounds and Phospholipids of Bacterial Cell Membrane on Model Monolayers. Molecules. 2014; 19(6):7497-7515. https://doi.org/10.3390/molecules19067497
Chicago/Turabian StyleNowotarska, Stella W., Krzysztof J. Nowotarski, Mendel Friedman, and Chen Situ. 2014. "Effect of Structure on the Interactions between Five Natural Antimicrobial Compounds and Phospholipids of Bacterial Cell Membrane on Model Monolayers" Molecules 19, no. 6: 7497-7515. https://doi.org/10.3390/molecules19067497
APA StyleNowotarska, S. W., Nowotarski, K. J., Friedman, M., & Situ, C. (2014). Effect of Structure on the Interactions between Five Natural Antimicrobial Compounds and Phospholipids of Bacterial Cell Membrane on Model Monolayers. Molecules, 19(6), 7497-7515. https://doi.org/10.3390/molecules19067497