Synthesis and Fungicidal Activity of Novel Chloro-Containing 1-Aryl-3-oxypyrazoles with an Oximino Ester or Oximino Amide Moiety
Abstract
:1. Introduction

2. Results and Discussion
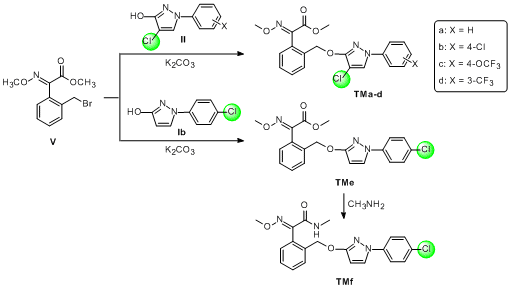
2.1. Chemistry



2.2. Fungicidal Activity
| Compounds | Structure | Rhizoctonia solani a | ||
|---|---|---|---|---|
| 10 μg/mL | 1 μg/mL | 0.1 μg/mL | ||
| TMa |  | +++ | +++ | +++ |
| TMb |  | +++ | +++ | + |
| TMe |  | +++ | +++ | +++ |
| TMf |  | +++ | +++ | ++ |
| TMc |  | ++ | + | + |
| TMd |  | ++ | +++ | ++ |
| Pyraclostrobin |  | ++ | + | + |
| Compounds | concn (μg/mL) a | EC90 (μg mL−1) | ||
|---|---|---|---|---|
| 10 | 1 | 0.1 | ||
| TMa | 100.00 | 98.23 | 83.40 | 0.20 |
| TMe | 100.00 | 100.00 | 98.94 | <0.1 |
| TMf | 100.00 | 94.70 | 71.40 | 0.32 |
3. Experimental
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. General Procedure for the Synthesis of IIa–IId
3.2.2. General Procedure for the Synthesis of III
3.2.3. General Procedure for the Synthesis of IV
3.2.4. General Procedure for the Synthesis of V
3.2.5. General Procedure for the Synthesis of TMa–TMe
3.2.6. General Procedure for the Synthesis of TMf
3.3. Fungicidal Activity Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clough, J.M.; Godfrey, C.R.A.; de Fraine, P.J. Fungicidal Aryloximinoacetates. EP Patent 472300A2, 26 February 1992. [Google Scholar]
- Zhao, P.-L.; Liu, C.-L.; Huang, W.; Wang, Y.-Z.; Yang, G.-F. Synthesis and fungicidal evaluation of novel chalcone-based strobilurin analogues. J. Agric. Food Chem. 2007, 55, 5697–5700. [Google Scholar] [CrossRef]
- Tu, S.; Xu, L.H.; Ye, L.Y.; Wang, X.; Sha, Y.; Xiao, Z.Y. Synthesis and fungicidal activities of novel indene-substituted oxime ether strobilurins. J. Agric. Food Chem. 2008, 56, 5247–5253. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, J.; Kim, H.; Kyung, S. Synthesis and SAR of methoxyiminoacetate and methoxyiminoacetamide derivatives as strobilurin analogues. Bull. Korean Chem. Soc. 2009, 30, 1475–1480. [Google Scholar]
- Herms, S.; Seehaus, K.; Koehle, H.; Conrath, U. A strobilurin fungicide enhances the resistance of tobacco against tobacco mosaic virus and Pseudomonas syringae pv tabaci. Plant Physiol. 2002, 130, 120–127. [Google Scholar] [CrossRef]
- Mercader, J.V.; Suarez-Pantaleon, C.; Agullo, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Production and characterization of monoclonal antibodies specific to the strobilurin pesticide pyraclostrobin. J. Agric. Food Chem. 2008, 56, 7682–7690. [Google Scholar] [CrossRef]
- Wolf, B.; Benoit, R.; Sauter, H.; Grammenos, W.; Wingert, H.; Kuekenhoehner, T.; Hepp, M. Preparation of E-methoximes of 2-(phenoxymethyl)phenylglyoxylates. DE Patent 4042272A1, 2 July 1992. [Google Scholar]
- Clough, J.M.; Godfrey, C.R.A.; Streeting, I.T.; Cheetham, R.; de Fraine, P.J.; Bartholomew, D.; Eshelby, J.J. Preparation of [(Pyrimidinyloxy)phenyl]methoxypropenoates and Related Compounds as Agrochemical Fungicides. EP Patent 468695A1, 29 January 1992. [Google Scholar]
- Liu, Y.; He, G.; Chen, K.; Li, Y.; Zhu, H. Synthesis, crystal structure, and fungicidal activity of novel 1,5-diaryl-1H-pyrazol-3-oxy derivatives containing oxyacetic acid or oxy(2-thioxothiazolidin-3-yl)ethanone moieties. J. Heterocycl. Chem. 2012, 49, 1370–1375. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Shi, H.; He, G.-K.; Song, G.-L.; Zhu, H.-J. Synthesis, crystal structures, and fungicidal activity of novel 1,5-diaryl-3-(glucopyranosyloxy)-1H-pyrazoles. Helv. Chim. Acta 2012, 95, 1645–1656. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, H.; Li, Y.; Zhu, H. Synthesis, crystal structure, and fungicidal activity of novel 1,5-diaryl-1H-pyrazol-3-oxyacetate derivatives. J. Heterocycl. Chem. 2010, 47, 897–902. [Google Scholar] [CrossRef]
- Mulla, M.S.; Darwazeh, H.A. Field and Laboratory Investigations on the Control of Susceptible and Resistant Pasture Mosquitoes. In Proceedings and Papers of the Thirty-seventh Annual Conference of the California Mosquito Control Association, Las Angeles, CA, USA, 27-29 January 1969; CMCA Press: Los Angeles, CA, USA, 1970; Volume 37, pp. 76–81. [Google Scholar]
- Stierli, D.; Daina, A.; Walter, H.; Tobler, H.; Rajan, R. Novel Microbiocides. WO Patent 2009012998A1, 29 June 2009. [Google Scholar]
- Li, Y.; Liu, R.; Yan, Z.; Zhang, X.; Zhu, H. Synthesis, crystal structure and fungicidal activities of new type oxazolidinone-based strobilurin analogues. Bull. Korean Chem. Soc. 2010, 31, 3341–3347. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Chen, K.; Jin, Y.; Li, Y.; Zhu, H. DMF-Catalyzed direct and regioselective C–H functionalization: Electrophilic/nucleophilic 4-halogenation of 3-oxypyrazoles. Eur. J. Org. Chem. 2011, 2011, 5323–5330. [Google Scholar] [CrossRef]
- Li, G.; Yang, H. Synthesis and antifungal bioactivity of methyl 2-methoxyimino-2-{2-[(substituted benzylidene)aminooxymethyl]phenyl}acetate and 2-methoxy imino-2-{2-[(substituted benzylidene)aminooxymethyl]phenyl}-N-methylacetamide derivatives. Chin. J. Chem. 2009, 27, 2055–2060. [Google Scholar] [CrossRef]
- Wilamowski, J.; Kulig, E.; Sepiol, J.J.; Burgiel, Z.J. Synthesis and in vitro antifungal activity of 1-amino-3,4-dialkylnaphthalene-2-carbonitriles and their analogues. Pest Manag. Sci. 2001, 57, 625–632. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the intermediates and target products are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Li, Y.; Chen, N.; Lv, K.; Zhou, C.; Xiong, X.; Li, F. Synthesis and Fungicidal Activity of Novel Chloro-Containing 1-Aryl-3-oxypyrazoles with an Oximino Ester or Oximino Amide Moiety. Molecules 2014, 19, 8140-8150. https://doi.org/10.3390/molecules19068140
Liu Y, Li Y, Chen N, Lv K, Zhou C, Xiong X, Li F. Synthesis and Fungicidal Activity of Novel Chloro-Containing 1-Aryl-3-oxypyrazoles with an Oximino Ester or Oximino Amide Moiety. Molecules. 2014; 19(6):8140-8150. https://doi.org/10.3390/molecules19068140
Chicago/Turabian StyleLiu, Yuanyuan, Yi Li, Nanqing Chen, Kunzhi Lv, Chao Zhou, Xiaohui Xiong, and Fangshi Li. 2014. "Synthesis and Fungicidal Activity of Novel Chloro-Containing 1-Aryl-3-oxypyrazoles with an Oximino Ester or Oximino Amide Moiety" Molecules 19, no. 6: 8140-8150. https://doi.org/10.3390/molecules19068140
APA StyleLiu, Y., Li, Y., Chen, N., Lv, K., Zhou, C., Xiong, X., & Li, F. (2014). Synthesis and Fungicidal Activity of Novel Chloro-Containing 1-Aryl-3-oxypyrazoles with an Oximino Ester or Oximino Amide Moiety. Molecules, 19(6), 8140-8150. https://doi.org/10.3390/molecules19068140