A PPARγ, NF-κB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic db/db Mice Liver
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Curcumin on Body Weight and Blood Glucose Levels

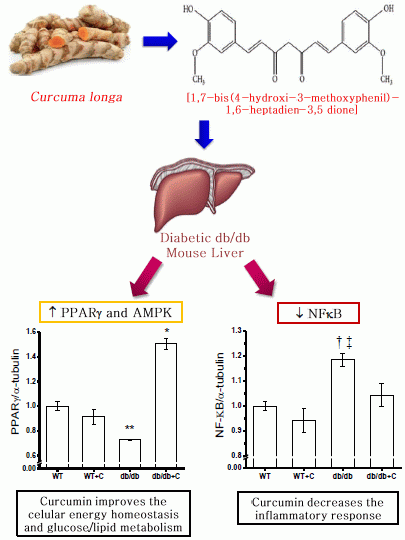
2.2. Effect of Curcumin on NF-κB Expression

2.3. Effect of Curcumin on AMPK and SIRT1 Expression

2.4. Effect of Curcumin on PGC-1α and PPARγ Expression

2.5. Effect of Curcumin on the Protein Nitration Content

3. Experimental
3.1. Reagents and Antibodies
3.2. Animals and Treatment
3.3. Liver Proteins Extraction
3.4. Western Immunobloting Assay
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2012, 39, 37–55. [Google Scholar] [CrossRef]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014, 80, 249–254. [Google Scholar]
- Shehzad, A.; Ha, T.; Subhan, F.; Lee, Y.S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 2011, 50, 151–161. [Google Scholar] [CrossRef]
- Bradford, P.G. Curcumin and obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Young-Joon, S.; Shishodia, S. Molecular targets and therapeutic uses of curcumin in health and disease. In Advances in Experimental Medicine and Biology; Bharat, B., Aggarwal, Y.J.S., Shishir, S., Eds.; Springer US: New York, NY, USA, 2007; Volume 595, pp. 227–243. [Google Scholar]
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and diabetes: A systematic review. Evid. Based Complement. Alternat. Med. 2013, 2013, 636–653. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2013, 6th ed. Available online: http://www.idf.org/diabetesatlas (accessed on 17 June 2014).
- Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014, 5, e996. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Perez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and liver disease. Biofactors 2013, 39, 88–100. [Google Scholar] [CrossRef]
- Martinez-Morua, A.; Soto-Urquieta, M.G.; Franco-Robles, E.; Zuniga-Trujillo, I.; Campos-Cervantes, A.; Perez-Vazquez, V.; Ramirez-Emiliano, J. Curcumin decreases oxidative stress in mitochondria isolated from liver and kidneys of high-fat diet-induced obese mice. J. Asian Nat. Prod. Res. 2013, 15, 905–915. [Google Scholar] [CrossRef]
- Franco-Robles, E.; Campos-Cervantes, A.; Murillo-Ortiz, B.O.; Segovia, J.; Lopez-Briones, S.; Vergara, P.; Perez-Vazquez, V.; Solis-Ortiz, M.S.; Ramirez-Emiliano, J. Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl. Physiol. Nutr. Metab. 2014, 39, 211–218. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor nf-kappa b is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3t3-l1 adipocytes and angiogenesis and obesity in c57/bl mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Lin, Y.; Yi, W.; Liang, Z.; Yan, J.; Wang, N.; Deng, C.; Zhang, S.; Li, Y.; et al. Sirt1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 2013, 65, 667–679. [Google Scholar] [CrossRef]
- Picard, F.; Auwerx, J. Ppar(gamma) and glucose homeostasis. Annu. Rev. Nutr. 2002, 22, 167–197. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of pgc-1alpha and sirt1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Ren, X.Y.; Li, Y.N.; Qi, J.S.; Niu, T. Peroxynitrite-induced protein nitration contributes to liver mitochondrial damage in diabetic rats. J. Diabetes Complicat. 2008, 22, 357–364. [Google Scholar] [CrossRef]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.; Jeon, S.M.; Lee, M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef]
- Coleman, D.L.; Hummel, K.P. Studies with the mutation, diabetes, in the mouse. Diabetologia 1967, 3, 238–248. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar] [CrossRef]
- Steigerwalt, R.; Nebbioso, M.; Appendino, G.; Belcaro, G.; Ciammaichella, G.; Cornelli, U.; Luzzi, R.; Togni, S.; Dugall, M.; Cesarone, M.R.; et al. Meriva(r), a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012, 54, 11–16. [Google Scholar]
- Dalboge, L.S.; Almholt, D.L.; Neerup, T.S.; Vassiliadis, E.; Vrang, N.; Pedersen, L.; Fosgerau, K.; Jelsing, J. Characterisation of age-dependent beta cell dynamics in the male db/db mice. PLoS One 2013, 8, e82813. [Google Scholar]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. Ikk-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of ikk-beta and nf-kappab. Nat. Med. 2005, 11, 183–190. [Google Scholar]
- Steinberg, G.R.; Kemp, B.E. Ampk in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate amp-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic ldl receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef]
- Oakhill, J.S.; Scott, J.W.; Kemp, B.E. Ampk functions as an adenylate charge-regulated protein kinase. Trends Endocrinol. Metab. 2012, 23, 125–132. [Google Scholar] [CrossRef]
- Yu, X.; McCorkle, S.; Wang, M.; Lee, Y.; Li, J.; Saha, A.K.; Unger, R.H.; Ruderman, N.B. Leptinomimetic effects of the amp kinase activator aicar in leptin-resistant rats: Prevention of diabetes and ectopic lipid deposition. Diabetologia 2004, 47, 2012–2021. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. Sirt1 regulates hepatocyte lipid metabolism through activating amp-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. Sirt1 modulation of the acetylation status, cytosolic localization, and activity of lkb1. Possible role in amp-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar]
- De Kreutzenberg, S.V.; Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Semplicini, A.; Dalla Man, C.; Cobelli, C.; Fadini, G.P.; Avogaro, A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: Potential biochemical mechanisms. Diabetes 2010, 59, 1006–1015. [Google Scholar] [CrossRef]
- Gillum, M.P.; Kotas, M.E.; Erion, D.M.; Kursawe, R.; Chatterjee, P.; Nead, K.T.; Muise, E.S.; Hsiao, J.J.; Frederick, D.W.; Yonemitsu, S.; et al. Sirt1 regulates adipose tissue inflammation. Diabetes 2011, 60, 3235–3245. [Google Scholar] [CrossRef]
- Fröjdö, S.; Durand, C.; Molin, L.; Carey, A.L.; El-Osta, A.; Kingwell, B.A.; Febbraio, M.A.; Solari, F.; Vidal, H.; Pirola, L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by sirt1. Mol. Cell. Endocrinol. 2011, 335, 166–176. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. Ampk and sirt1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Sansone, L.; Reali, V.; Pellegrini, L.; Villanova, L.; Aventaggiato, M.; Marfe, G.; Rosa, R.; Nebbioso, M.; Tafani, M.; Fini, M.; et al. Sirt1 silencing confers neuroprotection through igf-1 pathway activation. J. Cell. Physiol. 2013, 228, 1754–1761. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of sirt1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar]
- Lomb, D.J.; Laurent, G.; Haigis, M.C. Sirtuins regulate key aspects of lipid metabolism. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 2010, 1804, 1652–1657. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (pgc-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of pgc1 and nrf1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Wright, D.C.; Han, D.H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle pgc-1alpha expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar]
- Nandi, A.; Kitamura, Y.; Kahn, C.R.; Accili, D. Mouse models of insulin resistance. Physiol. Rev. 2004, 84, 623–647. [Google Scholar] [CrossRef]
- Galli, A.; Crabb, D.; Price, D.; Ceni, E.; Salzano, R.; Surrenti, C.; Casini, A. Peroxisome proliferator-activated receptor gamma transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology 2000, 31, 101–108. [Google Scholar] [CrossRef]
- Marra, F.; Efsen, E.; Romanelli, R.G.; Caligiuri, A.; Pastacaldi, S.; Batignani, G.; Bonacchi, A.; Caporale, R.; Laffi, G.; Pinzani, M.; et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology 2000, 119, 466–478. [Google Scholar] [CrossRef]
- Miyahara, T.; Schrum, L.; Rippe, R.; Xiong, S.; Yee, H.F., Jr.; Motomura, K.; Anania, F.A.; Willson, T.M.; Tsukamoto, H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J. Biol. Chem. 2000, 275, 35715–35722. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Y.; Kang, Q.; Feng, Y.; Chen, A. Curcumin inhibits gene expression of receptor for advanced glycation end-products (rage) in hepatic stellate cells in vitro by elevating ppargamma activity and attenuating oxidative stress. Br. J. Pharmacol. 2012, 166, 2212–2227. [Google Scholar] [CrossRef]
- Xu, J.; Fu, Y.; Chen, A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G20–G30. [Google Scholar]
- Ferroni, P.; Della-Morte, D.; Pileggi, A.; Riondino, S.; Rundek, T.; Ricordi, C.; Guadagni, F. Pleiotropic effects of ppargamma agonist on hemostatic activation in type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 2013, 11, 338–351. [Google Scholar] [CrossRef]
- Ishii, N.; Carmines, P.K.; Yokoba, M.; Imaizumi, H.; Ichikawa, T.; Ikenagasa, H.; Kodera, Y.; Oh-Ishi, M.; Aoki, Y.; Maeda, T.; et al. Angiotensin-converting enzyme inhibition curbs tyrosine nitration of mitochondrial proteins in the renal cortex during the early stage of diabetes mellitus in rats. Clin. Sci. 2013, 124, 543–552. [Google Scholar] [CrossRef]
- Kolodziejczyk, J.; Olas, B.; Saluk-Juszczak, J.; Wachowicz, B. Antioxidative properties of curcumin in the protection of blood platelets against oxidative stress in vitro. Platelets 2011, 22, 270–276. [Google Scholar] [CrossRef]
- Mythri, R.B.; Harish, G.; Dubey, S.K.; Misra, K.; Bharath, M.M. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: Implications for parkinson’s disease. Mol. cell. Biochem. 2011, 347, 135–143. [Google Scholar] [CrossRef]
- Hurkman, W.J.; Tanaka, C.K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986, 81, 802–806. [Google Scholar] [CrossRef]
- Encarnación, S.; Hernández, M.; Martínez-Batallar, G.; Contreras, S.; Vargas Mdel, C.; Mora, J. Comparative proteomics using 2-D gel electrophoresis and mass spectrometry as tools to dissect stimulons and regulons in bacteria with sequenced or partially sequenced genomes. Biol. Proced. Online 2005, 7, 117–135. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiménez-Flores, L.M.; López-Briones, S.; Macías-Cervantes, M.H.; Ramírez-Emiliano, J.; Pérez-Vázquez, V. A PPARγ, NF-κB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic db/db Mice Liver. Molecules 2014, 19, 8289-8302. https://doi.org/10.3390/molecules19068289
Jiménez-Flores LM, López-Briones S, Macías-Cervantes MH, Ramírez-Emiliano J, Pérez-Vázquez V. A PPARγ, NF-κB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic db/db Mice Liver. Molecules. 2014; 19(6):8289-8302. https://doi.org/10.3390/molecules19068289
Chicago/Turabian StyleJiménez-Flores, Lizbeth M., Sergio López-Briones, Maciste H. Macías-Cervantes, Joel Ramírez-Emiliano, and Victoriano Pérez-Vázquez. 2014. "A PPARγ, NF-κB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic db/db Mice Liver" Molecules 19, no. 6: 8289-8302. https://doi.org/10.3390/molecules19068289
APA StyleJiménez-Flores, L. M., López-Briones, S., Macías-Cervantes, M. H., Ramírez-Emiliano, J., & Pérez-Vázquez, V. (2014). A PPARγ, NF-κB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic db/db Mice Liver. Molecules, 19(6), 8289-8302. https://doi.org/10.3390/molecules19068289