Synthesis, Crystal Structure, DFT Studies and Evaluation of the Antioxidant Activity of 3,4-Dimethoxybenzenamine Schiff Bases
Abstract
:1. Introduction
(i) Proton Coupled-Electron Transfer (PC-ET) versus Hydrogen atom transfer (HAT)
(ii) Electron Transfer-Proton Transfer (ET-PT)
(iii) Sequential Proton Loss Electron Transfer (SPLET)
ArO− + R• → ArO• + R−
R− + H+ → RH
(iv) Adduct formation (AF)

2. Results and Discussion
2.1. Chemistry
2.2. Antioxidant Activities
2.2.1. DPPH Scavenging Activity
| N° | Yield (%) | IC50 (μM ± SEM a) | N° | Yield (%) | IC50 (μM ± SEM a) |
|---|---|---|---|---|---|
| 1 | 84 | 10.12 ± 0.54 | 14 | 87 | 42.80 ± 2.80 |
| 2 | 82 | 15.6 ± 0.06 | 15 | 82 | NA b |
| 3 | 78 | 19.2 ± 0.70 | 16 | 82 | NA b |
| 4 | 84 | 28.14 ± 0.86 | 17 | 84 | NA b |
| 5 | 85 | 30.45 ± 0.82 | 18 | 83 | NA b |
| 6 | 86 | 28.10 ± 1.30 | 19 | 85 | NA b |
| 7 | 81 | 33.02 ± 1.20 | 20 | 87 | NA b |
| 8 | 83 | 34.14 ± 1.50 | 21 | 88 | NA b |
| 9 | 92 | 40.01 ± 1.80 | 22 | - | NA b |
| 10 | 88 | NA b | 23 | 90 | NA b |
| 11 | 90 | 50.01 ± 2.20 | 24 | 84 | NA b |
| 12 | 87 | 38.16 ± 2.10 | 25 | 92 | NA b |
| 13 | 90 | NA b | n-propyl gallate c | - | 30.30 ± 0.2 |
2.2.2. Superoxide Scavenging Activity
| Comp. No. | IC50 (μM ± SEM a) | Comp. No | IC50 (μM ± SEM a) |
|---|---|---|---|
| 1 | 85.03 ± 1.20 | 14 | 260.3 ± 6.4 |
| 2 | 90.60 ± 1.50 | 15 | NA b |
| 3 | 98.60 ± 1.70 | 16 | NA b |
| 4 | 145 ± 2.1 | 17 | NA b |
| 5 | 170.2 ± 3.2 | 18 | NA b |
| 6 | 175.0 ± 3.5 | 19 | NA b |
| 7 | 180.1 ± 3.8 | 20 | 315.1 ± 8.4 |
| 8 | 190.1 ± 3.9 | 21 | 320.1 ± 6.3 |
| 9 | 208.9 ± 5.4 | 22 | NA b |
| 10 | NA b | 23 | NA b |
| 11 | NA b | 24 | NA b |
| 12 | 210.1 ± 4.4 | 25 | NA b |
| 13 | NA b | n-propyl gallate c | 106.34 ± 1.6 |
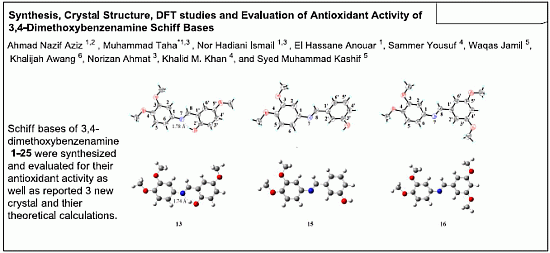
2.3. X-ray Crystallography Studies
2.3.1. Compound 13


| Compound 13 | Compound 15 | Compound 16 | |
|---|---|---|---|
| Empirical formula | C16H17NO4 | C15H15NO3 | C17H19NO4 |
| Formula weight | 287.31 | 257.28 | 301.33 |
| Temperature | 273(2)K | 273(2)K | 273(2)K |
| Wavelength | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Crystal system | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | Pna2(1) | P2(1)2(1)2(1) | P2(1)/c |
| a | 9.7203(7) Å | 5.3625(2)Å | 11.1898(4) Å |
| b | 30.576(2) Å | 11.1755(5) Å | 17.5567(6) Å |
| c | 4.8328(3) Å | 21.9532(10)Å | 8.1013(3) Å |
| α | 90° | 90° | 90° |
| β | 90° | 90° | 98.0720(10)° |
| γ | 90° | 90° | 90° |
| Volume | 1436.36(17)A3 | 1315.63(10)A3 | 1575.78(10) A3 |
| Z | 4 | 4 | 4 |
| Calculated density | 1.329 mg/m3 | 1.299 mg/m3 | 1.270 mg/m3 |
| Absorption coefficient | 0.096 mm−1 | 0.091 mm−1 | 0.091 mm−1 |
| F(000) | 608 | 544 | 640 |
| Crystal size | 0.67 × 0.16 × 0.14 mm | 0.77 × 0.49 × 0.45 mm | 0.46 × 0.44 × 0.42 mm |
| θ range | 1.33 to 25.50 ° | 1.86 to 25.50° | 1.84 to 25.50 |
| Reflections Collected | 8206 | 7839 | 9213 |
| Reflections Unique | 2629 | 2426 | 2934 |
| (Rint) | 0.0216 | 0.0165 | 0.0148 |
| R1 with I > 2σ(I) | 0.0347 | 0.0369 | 0.0352 |
| R2 with I > 2σ(I) | 0.0808 | 0.1066 | 0.0967 |
| R1 for all data | 0.0412 | 0.0386 | 0.0399 |
| R2 for all data | 0.0855 | 0.1086 | 0.1012 |
| Goodness of fit | 1.059 | 1.091 | 1.046 |
| max/min ρ eA°−3 | 0.107 and −0.129 | 0.349 and −0.295 | 0.140 and −0.141 |
| CCDC number | CCDC 980015 | CCDC 980014 | CCDC 980016 |
| D | H | A | D-H | H...A | D...A | D-H…A |
|---|---|---|---|---|---|---|
| O1' | H1A' | N7 | 0.94(3) | 1.78(3) | 2.628(2) | 149(2) |
| C5 | H5A | O2 a | 0.93 | 2.53 | 3.319(2) | 143 |
2.3.2. Compound 15
| D | H | A | D-H | H...A | D...A | D-H…A |
|---|---|---|---|---|---|---|
| O1' | H1A' | N7 a | 0.939(18) | 1.891(18) | 2.7841(16) | 158.1(16) |
| C6' | H6'A | O2 b | 0.93 | 2.58 | 3.2572(17) | 130 |
| C5 | H5A | O1 c | 0.93 | 2.57 | 3.1655(18) | 123 |
2.3.3. Compound 16
| D | H | A | D-H | H...A | D...A | D-H…A |
|---|---|---|---|---|---|---|
| C2 | H2’A | O1 a. | 0.93 | 2.57 | 3.4945(16) | 176 |
| C3 | H3A | O1 b | 0.93 | 2.52 | 3.4418(16) | 170 |
2.4. DFT Calculations

| 13 | 15 | 16 | Calculated | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal | Exp | Cal | Exp | Cal | Exp | 1 | 2 | 3 | 8 | 18 | |
| Bond lengths (Å) | |||||||||||
| C1-N7 | 1.41 | 1.413 | 1.40 | 1.4243 | 1.41 | 1.4181 | 1.40 | 1.41 | 1.41 | 1.40 | 1.41 |
| N7-C8 | 1.29 | 1.276 | 1.28 | 1.2673 | 1.28 | 1.2677 | 1.28 | 1.29 | 1.29 | 1.27 | 1.28 |
| C8-C1' | 1.45 | 1.452 | 1.47 | 1.4680 | 1.47 | 1.4650 | 1.47 | 1.44 | 1.45 | 1.46 | 1.47 |
| C2'-O2' | 1.34 | 1.358 | - | - | - | - | - | 1.37 | 1.35 | - | - |
| C3'-O3' | 1.37 | - | 1.37 | 1.3601 | 1.36 | 1.3673 | 1.36 | - | - | 1.37 | 1.36 |
| C5'-O4' | - | - | - | - | - | - | 1.37 | 1.36 | - | 1.36 | - |
| C5'-O5' | 1.37 | 1.382 | - | - | 1.37 | 1.3694 | 1.38 | - | 1.37 | - | - |
| C6'-O6' | - | - | - | - | - | - | - | 1.34 | - | - | - |
| Bond angles (°) | |||||||||||
| C2-C1-N7 | 125 | 123.31 | 126 | 122.15 | 126 | 122.79 | 123 | 123 | 123 | 123 | 123 |
| C1-N7-C8 | 124 | 121.07 | 123 | 119.72 | 123 | 118.20 | 121 | 122 | 122 | 120 | 121 |
| N7-C8-C1’ | 122 | 122.43 | 122 | 123.96 | 123 | 124.02 | 123 | 122 | 122 | 123 | 123 |
| Torsion angles (°) | |||||||||||
| C2-C1-N7-C8 | 0 | −155.52 | 0 | −43.09 | 0 | 34.70 | 37 | 32 | 34 | 36 | 30 |
| C1-N7-C8-C1' | −180 | −175.32 | −180 | 174.26 | −180 | −179.25 | −177 | −177 | −177 | −177 | −177 |
| N7-C8-C1'-C2’ | 0 | 0 | 0 | −1.1 | 0 | −174.83 | 2 | 0 | 1 | 1 | 1 |
3. Experimental
3.1. General Information
3.2. DPPH (1,1-Diphenyl-2-picryl hydrazyl) Free Radical Scavenging Activity
3.3. In Vitro Assay for Superoxide Anion Radical Scavenging Activity
3.4. General Procedure for the Synthesis 3,4-Dimethoxybenzenamine Schiff Bases
3.5. Theoretical Calculations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Rahim, F.; Jahun, H.; Perveen, S.; Choudhary, M.I. Synthesis of 2,4,6-trichlorophenyl hydrazones and their inhibitory potential against glycation of protein. Med. Chem. 2011, 7, 572–580. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Bibi, A.; Shahid, N.; Najam-ul-Haq, M.; Khan, M.; Taha, M.; Mughal, U.R.; Khan, K.M. Acylhydrazide and isatin Schiff bases as alternate UV-laser desorption ionization (LDI) matrices for low molecular weight (LMW) peptides analysis. Am. J. Anal. Chem. 2012, 3, 779–789. [Google Scholar] [CrossRef]
- Khan, K.M.; Khan, M.; Ali, M.; Taha, M.; Rasheed, S.; Perveen, S.; Choudhary, M.I. Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg. Med. Chem. 2009, 17, 7795–7780. [Google Scholar] [CrossRef]
- Khan, K.M.; Rahim, F.; Ambreen, N.; Taha, M.; Khan, M.; Jahan, H.; Najeebullah, U.; Shaikh, A.; Iqbal, S.; Perveen, S.; et al. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013, 9, 588–595. [Google Scholar] [CrossRef]
- Taha, M.; Naz, H.; Rasheed, S.; Ismail, N.H.; Rahman, A.A.; Yousuf, S.; Choudhary, M.I. Synthesis of 4-methoxybenzoylhydrazones and evaluation of their antiglycation activity. Molecules 2014, 19, 1286–1301. [Google Scholar] [CrossRef]
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; Choudhary, M.I. Acylhydrazide Schiff bases: Synthesis and antiglycation activity. J. Pak. Chem. Soc. 2013, 35, 929–937. [Google Scholar]
- Anouar, E.H.; Raweh, S.; Bayach, I.; Taha, M.; Baharudin, M.S.; Meo, F.D.; Hasan, M.H.; Adam, A.; Ismail, N.H.; Weber, J.F.; et al. Antioxidant properties of phenolic Schiff bases: Structure-activity relationship and mechanism of action. J. Comput. Aided Mol. Des. 2013, 27, 951–964. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2,4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar] [CrossRef]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Ali, S.; Perveen, S.; Choudhary, M.I.; Voelter, W. 2,4,6-Trichlorophenylhydrazine Schiff bases as dpph radical and super oxide anion scavengers. Med. Chem. 2012, 8, 452–461. [Google Scholar] [CrossRef]
- Khan, K.M.; Taha, M.; Naz, F.; Ali, S.; Perveen, S.; Choudhary, M.I. Synthesis of acylhydrazide Schiff bases and their anti-oxidant activity. Med. Chem. 2012, 8, 705–710. [Google Scholar] [CrossRef]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Khan, K.M.; Jaafar, F.M.; Samreen; Siddiqui, S.; Choudhary, M.I. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg. Med. Chem. Lett. 2013, 23, 3463–3466. [Google Scholar] [CrossRef]
- Sundriyal, S.; Sharma, R.K.; Jain, R. Current advances in antifungal targets and drug development. Curr. Med. Chem. 2006, 13, 1321–1335. [Google Scholar] [CrossRef]
- Popp, F.D.; Kirsch, W.J. Synthesis of potential anticancer agents. V. Schiff bases and related compounds. J. Org. Chem. 1961, 26, 3858–3860. [Google Scholar] [CrossRef]
- Jain, J.S.; Srivastava, R.S.; Aggarwal, N.; Sinha, R. Synthesis and evaluation of Schiff bases for anticonvulsant and behavioural depressant properties. Cent. Nerv. Syst. Agents Med. Chem. 2007, 7, 200–204. [Google Scholar] [CrossRef]
- Chinnasamy, R.P.; Sundararagan, R.; Govindaraj, S. Synthesis, characterization and analgesic activity of novel Schiff base isatin derivatives. Soc. Pharm. Edu. Res. 2010, 1, 342–347. [Google Scholar]
- Pandey, A.; Dewangan, D.; Verma, S.; Mishra, A.; Dubey, R.D. Synthesis of Schiff bases of 2-amino-5-aryl-1, 3,4-thiadiazole and its analgesic, anti-inflammatory, antibacterial and antitubercular activity. Int. J. Chem. Tech. Res. 2011, 3, 178–184. [Google Scholar]
- Mishra, P.; Gupta, P.N.; Shakya, A.K.; Shukla, R.; Srimal, R.C. Anti-inflammatory and diuretic activity of a new class of compounds-Schiff bases of 3-amino-2-methylquinazolin 4(3H)-ones. Indian J. Physiol. Pharmacol. 1995, 39, 169–172. [Google Scholar]
- Vicini, P.; Geronikaki, A.; Incerti, M.; Busonera, B.; Poni, G.; Cabras, C.A.; Colla, P.L. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg. Med. Chem. 2003, 11, 4785–4789. [Google Scholar] [CrossRef]
- Andreani, A.; Rambaldi, M.; Bonazzi, D.; Greci, L.; Andreani, F. Study on compounds with potential antitumor activity. III. Hydrazone derivatives of 5-substituted 2-chloro-3-formyl-6-methylindole. Farmaco Sci. 1979, 34, 132–138. [Google Scholar]
- Gemi, M.J.; Biles, C.; Keiser, B.J.; Poppe, S.M.; Swaney, S.M.; Tarapley, W.G.; Romeso, D.L.; Yage, Y. Novel 1,5-diphenylpyrazole nonnucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: Lead identification and SAR of 3- and 4-substituted derivatives. J. Med. Chem. 2000, 43, 1034–1040. [Google Scholar] [CrossRef]
- Satyanarayana, V.S.V.; Sivakumar, A.; Ghosh, A.R. Synthesis, characterization of some new five membered heterocycles based on imidazole moiety and their applications on therapeutics. Lett. Drug. Des. Discov. 2011, 8, 276–283. [Google Scholar] [CrossRef]
- Walcourt, A.; Loyevsky, M.; Lovejoy, D.B.; Gordeuk, V.R.; Richardson, D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004, 36, 401–407. [Google Scholar] [CrossRef]
- Gaur, S. Physico-chemical and Biological properties of Mn(II), Co(II), Ni(II) and Cu(II) chelates of Schiff Bases. Asian J. Chem. 2003, 15, 250–254. [Google Scholar]
- Nehru, K.; Athappan, P.; Rajagopal, G. Ruthenium(II)/(III) complexes of bidentate acetyl hydrazide Schiff bases. Transition Met. Chem. 2001, 26, 652–656. [Google Scholar] [CrossRef]
- Wen, X.; Hua-Xin, Z.; Zhong.-Lin, L.; Cheng.-Yong, S.; Bei.-Sheng, K. Classification, coordination and properties of acylhydrazone compounds. Zhongshan Daxue Xuebao 2001, 40, 39–43. [Google Scholar]
- Buu-Hoï, Ng.Ph.; Xuong, Ng.D.; Ham, Ng.H.; Binon, F.; Roger, R. Tuberculostatic hydrazides and their derivatives. J. Chem. Soc. 1953. [Google Scholar] [CrossRef]
- Ainscough, E.W.; Brodie, A.M.; Dobbs, A.J.; Ranford, J.D.; Waters, J.M. Antitumour copper(II) salicylaldehyde benzoyhydrazone (H2sb) complexes. Inorg. Chim. Acta 1998, 267, 27–38. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, N.; Fang, J.-G.; Tan, M.-Y. Synthesis, characterization and antioxidative activity of lanthanide complexes with 3,5-dibenz-yloxybenzoyl-2,4-dihydroxybenzaldehyde hydrazone. J. Chin. Rare Earth Soc. 2003, 21, 595–597. [Google Scholar]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.; Franco, D.; Dominguez, M.; Sineiro, J.; Dominguez, H.; Nunez, J. Natural antioxidants from residual sources, a review. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Hollman, P.C.; Hertog, M.G.; Katan, M.B. Analysis and health effects of flavonoids. Food Chem. 1996, 57, 43–46. [Google Scholar] [CrossRef]
- Schmidley, J.W. Free radicals in central nervous system ischemia. Stroke 1990, 21, 1086–1090. [Google Scholar]
- Meyer, A.S.; Heiononen, M.; Frankel, E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998, 61, 71–75. [Google Scholar] [CrossRef]
- Hunt, E.J.; Lester, C.E.; Lester, P.A.; Tackett, R.L. Effect of St. John's wort on free radical production. Life Sci. 2001, 69, 181–190. [Google Scholar] [CrossRef]
- Mayer, J.M.; Hrovat, D.A.; Thomas, J.L.; Borden, W.T. Proton-Coupled Electron Transfer versus Hydrogen Atom Transfer in Benzyl/Toluene, Methoxyl/Methanol, and Phenoxyl/Phenol Self-Exchange Reactions. J. Am. Chem. Soc. 2002, 124, 11142–11147. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Proton-coupled electron transfer: Classification scheme and guide to theoretical methods. Energy Environ. Sci. 2012, 5, 7696–7703. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Theoretical perspectives on proton-coupled electron transfer reactions. Acc. Chem. Res. 2001, 34, 273–281. [Google Scholar] [CrossRef]
- Anouar, E.H.; Shah, S.A.A.; Hassan, N.B.; Moussaoui, N.E.; Ahmad, R.; Zulkefeli, M.; Weber, J.-F.F. Antioxidant activity of hispidin oligomers from medicinal fungi: A DFT study. Molecules 2014, 19, 3489–3507. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.R.M.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Musialik, M.; Litwinienko, G. Scavenging of dpph* radicals by vitamin E is accelerated by its partial ionization: The role of sequential proton loss electron transfer. Org. Lett. 2005, 7, 4951–4954. [Google Scholar] [CrossRef]
- Khan, K.M.; Naz, F.; Taha, M.; Khan, A.; Perveen, S.; Choudhary, M.I.; Voelter, W. Synthesis and in vitro urease inhibitory activity of N,N'-disubsituted thioureas. Eur. J. Med. Chem. 2014, 74, 314–323. [Google Scholar] [CrossRef]
- Khan, K.M.; Jamil, W.; Ambreen, N.; Taha, M.; Perveen, S.; Morales, G.A. An Expeditious synthetic approach towards the synthesis of bis-Schiff bases (aldazines) using ultrasound. Ultrason. Sonochem. 2014, 21, 1200–1205. [Google Scholar] [CrossRef]
- Khan, K.M.; Khan, M.; Ambreen, N.; Taha, M.; Rahim, F.; Rasheed, S.; Saied, S.; Shafi, H.; Perveen, S.; Choudhary, M.I. Oxindole derivatives: Synthesis and antiglycation activity. Med. Chem. 2013, 9, 681–688. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jaafar, F.M.; Aziz, A.N.; Yousuf, S. (E)-N′-(3,4-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide monohydrate. Acta Cryst. 2013, E69, o490. [Google Scholar]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Shah, S.A.A.; Yousuf, S. (E)-2-Methoxy-N′-(2,4,6-trihydroxybenzylidene)Benzohydrazide. Acta Cryst. 2013, E69, o277. [Google Scholar]
- Taha, M.; Ismail, N.H.; Jaafar, F.M.; Khan, K.M.; Yousuf, S. (E)-2,4-Dimethyl-N′-(2-methylbenzylidene) benzohydrazide. Acta Cryst. 2013, E69, o400. [Google Scholar]
- Csaszar, J. Spectral studies of molecular complexes of aromatic Schiff bases with picric acid. Acta Chim. Hung. 1990, 127, 277–286. [Google Scholar]
- Csaszar, J.; Balog, J. Spectra of aromatic Schiff bases. Acta Chim. Hung. 1975, 86, 100–116. [Google Scholar]
- Akkurt, M.; Jarrahpour, A.; Aye, M.; Gencaslan, M.; Buyuekgungor, O. 3,4-Dimethoxy-N-(4-nitrobenzylidene)aniline. Acta Cryst. E. 2008, 64, o2087. [Google Scholar]
- Yang, H.; Carter, R.G. Asymmetric construction of nitrogen-containing [2.2.2] Bicyclic Scaffolds Using N-(p-Dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide. J. Org. Chem. 2009, 74, 5151–5156. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Thangapandiyan, S.; Miltonprabu, S. An in vivo and in vitro studies on the antioxidant property of epigallocatechin gallate on sodium fluoride induced toxicity in rats. Int. J. Phytopharmacol. 2013, 4, 245–254. [Google Scholar]
- Barontini, M.; Bernini, R.; Crisante, F.; Fabrizi, G. Selective and efficient oxidative modifications of flavonoids with 2-iodoxybenzoic acid (IBX). Tetrahedron 2010, 66, 6047–6053. [Google Scholar] [CrossRef]
- Bernini, R.; Fabrizi, G.; Pouysegu, L.; Deffieux, D.; Quideau, S. Synthesis of biologically active catecholic compounds via ortho-selective oxygenation of phenolic compounds using hypervalent iodine(V) reagents. Curr. Org. Synth. 2012, 9, 650–669. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Fabrizi, G.; Gentili, P. Convenient synthesis of 1-aryl-dihydroxyisochromans exhibiting antioxidant activity. Curr. Org. Chem. 2012, 16, 1051–1057. [Google Scholar] [CrossRef]
- Barontini, M.; Bernini, R.; Carastro, R.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816. [Google Scholar] [CrossRef]
- Perez-Gonzalez, A.; Galano, A.; Alvarez-Idaboy, J.R. Dihydroxybenzoic acids as free radical scavengers: Mechanisms, kinetics, and trends in activity. New J. Chem. 2014, 38, 2639–2652. [Google Scholar] [CrossRef]
- Marković, Z.; Đorović, J.; Dimitrić Marković, J.; Živić, M.; Amić, D. Investigation of the radical scavenging potency of hydroxybenzoic acids and their carboxylate anions. Monatsh. Chem. Chem. Mon. 2014, 145, 953–962. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aziz, A.N.; Taha, M.; Ismail, N.H.; Anouar, E.H.; Yousuf, S.; Jamil, W.; Awang, K.; Ahmat, N.; Khan, K.M.; Kashif, S.M. Synthesis, Crystal Structure, DFT Studies and Evaluation of the Antioxidant Activity of 3,4-Dimethoxybenzenamine Schiff Bases. Molecules 2014, 19, 8414-8433. https://doi.org/10.3390/molecules19068414
Aziz AN, Taha M, Ismail NH, Anouar EH, Yousuf S, Jamil W, Awang K, Ahmat N, Khan KM, Kashif SM. Synthesis, Crystal Structure, DFT Studies and Evaluation of the Antioxidant Activity of 3,4-Dimethoxybenzenamine Schiff Bases. Molecules. 2014; 19(6):8414-8433. https://doi.org/10.3390/molecules19068414
Chicago/Turabian StyleAziz, Ahmad Nazif, Muhammad Taha, Nor Hadiani Ismail, El Hassane Anouar, Sammer Yousuf, Waqas Jamil, Khalijah Awang, Norizan Ahmat, Khalid M. Khan, and Syed Muhammad Kashif. 2014. "Synthesis, Crystal Structure, DFT Studies and Evaluation of the Antioxidant Activity of 3,4-Dimethoxybenzenamine Schiff Bases" Molecules 19, no. 6: 8414-8433. https://doi.org/10.3390/molecules19068414
APA StyleAziz, A. N., Taha, M., Ismail, N. H., Anouar, E. H., Yousuf, S., Jamil, W., Awang, K., Ahmat, N., Khan, K. M., & Kashif, S. M. (2014). Synthesis, Crystal Structure, DFT Studies and Evaluation of the Antioxidant Activity of 3,4-Dimethoxybenzenamine Schiff Bases. Molecules, 19(6), 8414-8433. https://doi.org/10.3390/molecules19068414