Studies on the Anticonvulsant Activity and Influence on GABA-ergic Neurotransmission of 1,2,4-Triazole-3-thione- Based Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry


2.2. Pharmacology
2.2.1. Anticonvulsant Activity
| Compounds | Pretreatment Time (min) | |||
|---|---|---|---|---|
| 15 | 30 | 60 | 120 | |
| p/t (%) | p/t (%) | p/t (%) | p/t (%) | |
| 1a | 0/8 (0) | 0/8 (0) | 1/8 (12.5) | 1/8 (12.5) |
| 2a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 3a | * | * | * | * |
| 4a | 8/8 (100) | 8/8 (100) | 7/8 (87.5) | 7/8 (87.5) |
| 5a | 0/8 (0) | 0/8 (0) | 1/8 (12.5) | 1/8 (12.5) |
| 6a | 4/8 (50) | 6/8 (75) | 6/8 (75) | 3/8 (37.5) |
| 7a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 8a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 9a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 1/8 (12.5) |
| 10a | 8/8 (100) | 8/8 (100) | 8/8 (100) | 6/8 (75) |
| 11a | 1/8 (12.5) | 1/8 (12.5) | 1/8 (12.5) | 0/8 (0) |
| 12a | 6/8 (75) | 6/8 (75) | 7/8 (87.5) | 7/8 (87.5) |
| 13a | 8/8 (100) | 8/8 (100) | 8/8 (100) | 8/8 (100) |
| 14a | * | * | * | * |
| 15a | 6/8 (75) | 8/8 (100) | 8/8 (100) | 7/8 (87.5) |
| 16a | 5/8 (62.5) | 7/8 (87.5) | 8/8 (100) | 8/8 (100) |
| 17a | 0/8 (0) | 0/8 (0) | 1/8 (12.5) | 0/8 (0) |
| 18a | 0/8 (0) | 0/8 (0) | 1/8 (12.5) | 0/8 (0) |
| 19a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 20a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 21a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 22a | 4/8 (50) | 5/8 (62.5) | 3/8 (37.5) | 2/8 (25) |
| 23a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 24a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 25a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 26a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 27a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 28a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 29a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| 30a | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| Compounds | PretreatmentTime (min) | ED50 ± S.E.(mg/kg) | n | TD50 ± S.E.(mg/kg) | n | PI (TD50/ED50) |
|---|---|---|---|---|---|---|
| 3a | 15 | 35.2 ± 5.3 | 8 | 136.7 ± 19.9 | 24 | 3.9 |
| 30 | 93.6 ± 9.4 | 16 | 148.8 ± 19.9 | 32 | 1.6 | |
| 60 | >200 | - | 171.3 ± 21.4 | 24 | - | |
| 120 | >200 | - | 201.0 ± 20.3 | 32 | - | |
| 4a | 15 | 90.1 ± 15.7 | 16 | 354.9 ± 21.5 | 24 | 3.9 |
| 30 | 135.0 ± 19.8 | 8 | 365.3 ± 24.0 | 16 | 2.7 | |
| 60 | 171.3 ± 21.4 | 24 | 361.0 ± 20.2 | 32 | 2.1 | |
| 120 | 183.8 ± 28.0 | 24 | 375.8 ± 26.1 | 32 | 2.0 | |
| 6a | 15 | 297.5 ± 21.1 | 24 | 372.5 ± 22.6 | 16 | 1.2 |
| 30 | 217.8 ± 21.7 | 16 | 383.7 ± 18.7 | 16 | 1.8 | |
| 60 | 208.9 ± 27.3 | 32 | 389.3 ± 19.1 | 16 | 1.9 | |
| 120 | 335.7 ± 14.0 | 32 | 424.7 ± 21.4 | 32 | 1.3 | |
| 10a | 15 | 57.0 ± 9.4 | 16 | 338.1 ± 12.0 | 24 | 5.9 |
| 30 | 74.5 ± 8.1 | 16 | 338.1 ± 14.7 | 16 | 4.5 | |
| 60 | 187.1 ± 18.8 | 16 | 333.4 ± 18.6 | 16 | 1.8 | |
| 120 | 281.4 ± 13.6 | 32 | 395.1 ± 25.2 | 24 | 1.4 | |
| 12a | 15 | 207.5 ± 25.3 | 32 | 534.3 ± 25.9 | 40 | 2.6 |
| 30 | 186.4 ± 24.6 | 32 | 496.8 ± 31.7 | 32 | 2.7 | |
| 60 | 217.8 ± 21.7 | 16 | 418.5 ± 34.3 | 32 | 1.9 | |
| 120 | 208.9 ± 27.3 | 32 | 483.8 ± 25.9 | 40 | 2.3 | |
| 13a | 15 | 104.0 ± 15.8 | 8 | 195.7 ± 21.8 | 8 | 1.9 |
| 30 | 100.0 ± 30.1 | 8 | 191.2 ± 21.1 | 8 | 1.9 | |
| 60 | 108.9 ± 11.9 | 16 | 176.1 ± 17.2 | 16 | 1.6 | |
| 120 | 80.0 ± 10.9 | 24 | 217.8 ± 21.7 | 16 | 2.7 | |
| 15a | 15 | 182.7 ± 25.1 | 24 | 278.9 ± 34.8 | 32 | 1.5 |
| 30 | 104.7 ± 16.5 | 24 | 250.0 ± 26.9 | 24 | 2.4 | |
| 60 | 136.7 ± 19.9 | 24 | 247.9 ± 39.5 | 32 | 1.8 | |
| 120 | 175.5 ± 25.0 | 24 | 240.9 ± 27.3 | 24 | 1.4 | |
| 16a | 15 | 272.5 ± 21.4 | 32 | 430.9 ± 27.9 | 32 | 1.6 |
| 30 | 181.7 ± 44.3 | 8 | 471.7 ± 26.7 | 32 | 2.6 | |
| 60 | 152.6 ± 23.5 | 24 | 455.8 ± 21.7 | 24 | 3.0 | |
| 120 | 140.9 ± 25.1 | 16 | 404.4 ± 24.0 | 24 | 2.9 | |
| 22a | 15 | 223.5 ± 20.9 | 24 | 341.0 ± 24.8 | 32 | 1.5 |
| 30 | 228.3 ± 14.7 | 16 | 310.2 ± 26.2 | 32 | 1.4 | |
| 60 | 274.2 ± 10.9 | 8 | 433.2 ± 26.5 | 32 | 1.6 | |
| 120 | 448.1 ± 21.4 | 24 | 455.5 ± 29.7 | 32 | 1.0 | |
| Valproate | 15 | 189.0 ± 17.3 | 24 | 363.3 ± 14.2 | 24 | 1.9 |
| 30 | 216.9 ± 9.4 | 16 | 372.9 ± 16.9 | 16 | 1.7 | |
| 60 | 218.4 ± 18.9 | 24 | 417.3 ± 9.5 | 16 | 1.9 | |
| 120 | 246.6 ± 21.6 | 24 | 512.3 ± 20.2 | 32 | 2.1 |
| Compounds | MW | logP | TPSA | ABS (%) |
|---|---|---|---|---|
| 1a | 305.765 | 3.817 | 33.617 | 97.40 |
| 2a | 305.765 | 3.841 | 33.617 | 97.40 |
| 3a | 305.765 | 3.654 | 33.617 | 97.40 |
| 4a | 322.22 | 4.168 | 33.617 | 97.40 |
| 5a | 366.671 | 4.462 | 33.617 | 97.40 |
| 6a | 366.671 | 4.299 | 33.617 | 97.40 |
| 7a | 413.671 | 4.573 | 33.617 | 97.40 |
| 8a | 301.802 | 4.102 | 33.617 | 97.40 |
| 9a | 301.802 | 4.126 | 33.617 | 97.40 |
| 10a | 301.802 | 3.938 | 33.617 | 97.40 |
| 11a | 329.812 | 3.388 | 50.688 | 91.51 |
| 12a | 305.765 | 3.841 | 33.617 | 97.40 |
| 13a | 323.755 | 3.981 | 33.617 | 97.40 |
| 14a | 356.665 | 5.009 | 33.617 | 97.40 |
| 15a | 366.671 | 4.486 | 33.617 | 97.40 |
| 16a | 366.671 | 4.32 | 33.617 | 97.40 |
| 17a | 333.869 | 3.947 | 33.617 | 97.40 |
| 18a | 358.898 | 4.368 | 36.855 | 96.28 |
| 19a | 465.804 | 4.23 | 35.232 | 96.84 |
| 20a | 540.918 | 5.973 | 29.236 | 98.91 |
| 21a | 558.908 | 6.136 | 29.236 | 98.91 |
| 22a | 400.935 | 3.869 | 35.232 | 96.84 |
| 23a | 476.049 | 5.612 | 29.236 | 98.91 |
| 24a | 494.039 | 5.776 | 29.236 | 98.91 |
| 25a | 465.804 | 4.417 | 35.232 | 96.84 |
| 26a | 540.918 | 6.16 | 29.236 | 98.91 |
| 27a | 558.908 | 6.324 | 29.236 | 98.91 |
| 28a | 465.804 | 4.254 | 35.232 | 96.84 |
| 29a | 540.918 | 5.997 | 29.236 | 98.91 |
| 30a | 558.908 | 6.16 | 29.236 | 98.91 |
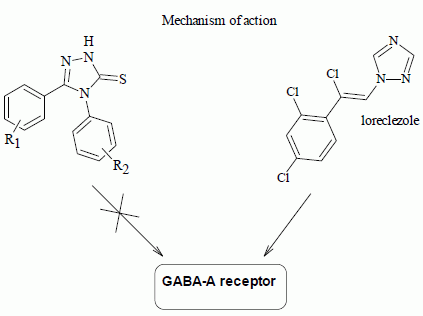
2.2.2. Radioligand Binding Assay
| Compounds (Concentration 1 × 10−6 M) | Percent of Specific Binding (%) | |
|---|---|---|
| GABAA | BDZ | |
| 1a | 8 | 14 |
| 5a | 2 | 12 |
| 6a | 17 | 20 |
| 10a | 2 | 9 |
| 12a | 13 | 18 |
| 15a | 8 | 21 |
| 16a | 17 | 24 |
| 17a | 21 | 6 |
| 22a | 6 | 12 |
| 23a | 23 | 7 |
| 24a | 21 | 13 |
| GABA | 82 | - |
| Diazepam | - | 83 |
| Zolpidem | - | 73 |
| Treatment | Ki ± S.E. [nM] | IC50 ± S.E. [nM] |
|---|---|---|
| GABA | 96.6 ± 9.9 | 406.5 ± 42.0 |
| GABA + 6a (1 × 10−5 M) | 99.7 ± 11.6 | 370.3 ± 42.7 |
| GABA + 6a (1 × 10−6 M) | 108.0 ± 11.2 | 469.0 ± 55.9 |
| GABA + 6a (1 × 10−7 M) | 155.0 ± 16.5 | 496.0 ± 57.0 |
| GABA + 10a (1 × 10−5 M) | 112.3 ± 8.5 | 474.0 ± 36.2 |
| GABA + 10a (1 × 10−6 M) | 85.5 ± 1.5 | 358.5 ± 6.5 |
| GABA + 10a (1 × 10−7 M) | 107.0 ± 5.0 | 452.0 ± 22.0 |
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Thiosemicarbazide Derivatives (1–18)
3.1.2. General Procedure for the Synthesis of 1,2,4-triazole-3-thione Derivatives (1a–18a)
3.1.3. General Procedure for the Synthesis of Mannich Bases (19a–30a)
3.2. Pharmacology
3.2.1. General Information
3.2.2. Maximal Electroshock Seizure Test
3.2.3. Chimney Test
3.2.4. Protective Index (PI)
3.2.5. Radioligand Binding Assay
| Receptor | Radioligand | Blank(nonspecific) | Buffer | Incubation Conditions |
|---|---|---|---|---|
| GABAA | [3H]muscimol | 100 µM GABA | 50 mM Tris-HCl pH 7.4 | 10 min, 0–4 °C |
| BDZ | [3H]flunitrazepam | 10 µM diazepam | 50 mM Tris-HCl pH 7.4 | 20 min, 0–4 °C |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Savage, N. Epidemiology: The complexities of epilepsy. Nature 2014, 511, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.T.; Jallon, P.; Preux, P.M. The epidemiology of seizure disorders in infancy and childhood: Definitions and classifications. Handb. Clin. Neurol. 2013, 111, 391–398. [Google Scholar] [PubMed]
- Heja, L. Astrocytic target mechanisms in epilepsy. Curr. Med. Chem. 2014, 21, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.M.; Rogawski, M.A.; Klitgaard, H. The potential of antiseizure drugs and agents that act on novel molecular targets as antiepileptogenic treatments. Neurotherapeutics 2014, 11, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S.; Rogawski, M.A. Molecular targets for antiepileptic drug development. Neurotherapeutics 2007, 4, 18–61. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, M.; Curia, G.; Biagini, G.; Ragsdale, D.S.; Avioli, M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010, 9, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Lipkind, G.M.; Fozzard, H.A. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol. Pharmacol. 2010, 78, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Malawska, B. Application of pharmacophore models for the design and synthesis of new anticonvulsant drugs. Mini Rev. Med. Chem. 2003, 3, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Yogeeswari, P.; Stables, J.P. Synthesis and anticonvulsant activity of 4-bromophenyl substituted aryl semicarbazones. Eur. J. Med. Chem. 2000, 35, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Sriram, D.; Jit, L.R.J.S.; Kumar, S.S.; Stables, J.P. Anticonvulsant and neurotoxicity evaluation of some 6-chlorobenzothiazolyl-2-thiosemicarbazones. Eur. J. Med. Chem. 2002, 37, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, P.B.; Wafford, K.A.; Bain, C.; Whiting, P.J. The modulatory action of loreclezole at the γ-aminobutyric acid type A receptor is determined by a single amino acid in the β2 subunit. Proc. Natl. Acad. Sci. USA 1994, 91, 4569–4573. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Plech, T.; Wujec, M. Effect of 4-(4-bromophenyl)-5-(3-chlorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione on the anticonvulsant action of different classical antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Eur. J. Pharmacol. 2012, 690, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Plech, T.; Wujec, M. Influence of 5-(3-chlorophenyl)-4-(4-methylphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione on the anticonvulsant action of four classical antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Pharmacol. Rep. 2012, 64, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Shalini, M.; Yogeeswari, P.; Sriram, D.; Stables, J.P. Cyclization of the semicarbazone template of aryl semicarbazones: Synthesis and anticonvulsant activity of 4,5-diphenyl-2H-1,2,4-triazol-3(4H)-one. Biomed. Pharmacother. 2009, 63, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Ahsan, W. Triazole incorporated thiazoles as a new class of anticonvulsants: Design, synthesis and in vivo screening. Eur. J. Med. Chem. 2010, 45, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Almasirad, A.; Tabatabai, S.A.; Faizi, M.; Kebriaeezadeh, A.; Mehrabi, N.; Dalvandi, A.; Shafiee, A. Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg. Med. Chem. Lett. 2004, 14, 6057–6059. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, T.; Tabatabai, S.A.; Khoshnoud, M.J.; Shafaghi, B.; Shafiee, A. Design and synthesis of 4H-3-(2-phenoxy)phenyl-1,2,4-triazole derivatives as benzodiazepine receptor agonists. Bioorg. Med. Chem. 2003, 11, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Wujec, M.; Siwek, A.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011, 46, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Almajan, G.L.; Barbuceanu, S.F.; Almajan, E.R.; Draghici, C.; Saramet, G. Synthesis, characterization and antibacterial activity of some triazole Mannich bases carrying diphenylsulfone moieties. Eur. J. Med. Chem. 2009, 44, 3083–3089. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H.; Camenish, G.; Folkers, G.; Chretien, J.R.; Raevsky, O.A. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding characteristics. J. Drug Target. 1998, 6, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Palm, K.; Luthman, K.; Ungell, A.L.; Strandlund, G.; Artursson, P. Correlation of drug absorption with molecular surface properties. J. Pharm. Sci. 1996, 85, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Abraham, M.H.; Lee, J.; Hersey, A.; Luscombe, N.C.; Beck, G.; Sherborne, B.; Cooper, I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Chebib, M.; Johnston, G.A.R. The ABC of GABA receptors: A brief review. Clin. Exp. Pharmacol. Physiol. 1999, 26, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Czuczwar, S.J.; Patsalos, P.N. The new generation of GABA enhancers. CNS Drugs 2001, 15, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Czapiński, P.; Blaszczyk, B.; Czuczwar, S.J. Mechanisms of action of antiepileptic drugs. Curr. Top. Med. Chem. 2005, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Łuszczki, J.J.; Wujec, M.; Flieger, J.; Pizoń, M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Pizoń, M.; Plech, T.; Łuszczki, J.J. Analysis of new potential anticonvulsant compounds in mice brain tissue by SPE/HPLC/DAD. J. Chromatogr. B 2012, 909, 26–33. [Google Scholar]
- Feng, H.J. Allosteric modulation of αβδ GABAA receptors. Pharmaceuticals 2010, 3, 3461–3477. [Google Scholar] [CrossRef]
- Wafford, K.A.; Bain, C.J.; Quirk, K.; McKernan, R.M.; Wingrove, P.B.; Whiting, P.J.; Kemp, J.A. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron 1994, 12, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration. Property Calculator. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 15 March 2014).
- Plech, T.; Wujec, M.; Majewska, M.; Kosikowska, U.; Malm, A. Microbiologically active Mannich bases derived from 1,2,4-triazoles. The effect of C-5 substituent on antibacterial activity. Med. Chem. Res. 2013, 22, 2531–2537. [Google Scholar]
- Plech, T.; Wujec, M.; Kaproń, B.; Kosikowska, U.; Malm, A. Synthesis and antibacterial activity of some novel N2-hydroxymethyl and N2-aminomethyl derivatives of 4-aryl-5-(3-chlorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione. Heteroat. Chem. 2011, 22, 737–743. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Boissier, J.R.; Tardy, J.; Diverres, J.C. Une nouvelle méthode simple pour explorer l’action tranquilisante: Le test de la cheminée. Med. Exp. 1960, 3, 81–84. [Google Scholar]
- Löscher, W.; Nolting, B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs IV. Protective indices. Epilepsy Res. 1991, 9, 1–10. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Plech, T.; Kaproń, B.; Łuszczki, J.J.; Wujec, M.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Żołnierek, M.; Nowak, G. Studies on the Anticonvulsant Activity and Influence on GABA-ergic Neurotransmission of 1,2,4-Triazole-3-thione- Based Compounds. Molecules 2014, 19, 11279-11299. https://doi.org/10.3390/molecules190811279
Plech T, Kaproń B, Łuszczki JJ, Wujec M, Paneth A, Siwek A, Kołaczkowski M, Żołnierek M, Nowak G. Studies on the Anticonvulsant Activity and Influence on GABA-ergic Neurotransmission of 1,2,4-Triazole-3-thione- Based Compounds. Molecules. 2014; 19(8):11279-11299. https://doi.org/10.3390/molecules190811279
Chicago/Turabian StylePlech, Tomasz, Barbara Kaproń, Jarogniew J. Łuszczki, Monika Wujec, Agata Paneth, Agata Siwek, Marcin Kołaczkowski, Maria Żołnierek, and Gabriel Nowak. 2014. "Studies on the Anticonvulsant Activity and Influence on GABA-ergic Neurotransmission of 1,2,4-Triazole-3-thione- Based Compounds" Molecules 19, no. 8: 11279-11299. https://doi.org/10.3390/molecules190811279
APA StylePlech, T., Kaproń, B., Łuszczki, J. J., Wujec, M., Paneth, A., Siwek, A., Kołaczkowski, M., Żołnierek, M., & Nowak, G. (2014). Studies on the Anticonvulsant Activity and Influence on GABA-ergic Neurotransmission of 1,2,4-Triazole-3-thione- Based Compounds. Molecules, 19(8), 11279-11299. https://doi.org/10.3390/molecules190811279