A One-Pot Approach to Pyridyl Isothiocyanates from Amines
Abstract
:1. Introduction

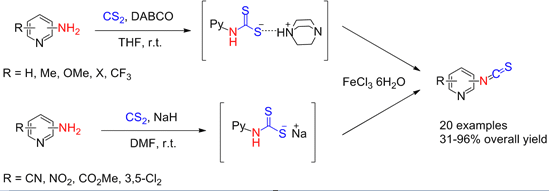
2. Results and Discussion

| Entry | Solvent | Base | PKa b | PKip c | Conversion of 1g (%) | Overall Yield (%) |
|---|---|---|---|---|---|---|
| 1 | THF | K2CO3 | 10.3 | 31 | 11 | |
| 2 | THF | KOH | 15.7 | 46 | 25 | |
| 3 | THF | pyridine | 5.4 | 2.2 | 0 | trace |
| 4 | THF | Proton sponge | 12.1 | 0 | trace | |
| 5 | THF | Et3N | 10.7 | 2.1 | 85 | 77 |
| 6 | THF | t-BuOK | 29.0 | 78 | trace | |
| 7 | THF | DABCO | 8.7 | 0.8 | 100 | 96 |
| 8 | THF | DBU | 11.6 | −3.8 | 100 | 90 |
| 9 | THF | DMAP | 9.9 | 0.61 | 100 | 90 |
| 10 | DMF | DABCO | 95 | 87 | ||
| 11 | acetone | DABCO | 86 | 70 | ||
| 12 | MeCN | DABCO | 84 | 70 | ||
| 13 | EtOH | DABCO | 0 | trace | ||
| 14 | CH2Cl2 | DABCO | 60 | 48 |
| Entry | Amines | Product | CS2 (equiv) | Time (h) b | Overall Yield (%) |
|---|---|---|---|---|---|
 |  | ||||
| 1 | R = H | 4a | 3 | 4 | 87 |
| 2 | R = Me | 4b | 3 | 4 | 88 |
 |  | ||||
| 3 | R = F | 4c | 3 | 12 | 76 |
| 4 | R = Cl | 4d | 10 | 12 | 81 |
| 5 | R = Br | 4e | 10 | 12 | 83 |
| 6 | R = CF3 | 4f | 20 | 24 | 42 |
| 7 |  | 4g | 3 | 4 | 96 |
| 8 |  | 4h | 3 | 2 | 91 |
| 9 |  | 4i | 10 | 12 | 73 |
 |  | ||||
| 10 | R = CN | 4j | 4 | 12 | 87 |
| 11 | R = NO2 | 4k | 5 | 24 | 77 |
| 12 | R = CF3 | 4l | 4 | 12 | 85 |
| 13 |  | 4m | 4 | 12 | 66 |
| Entry | Amine | Product | Overall Yield (%) |
|---|---|---|---|
 |  | ||
| 1 | R1 = H, R2 = CN | 4n | 51 |
| 2 | R1 = H, R2 = NO2 | 4o | 31 |
| 3 | R1 = H, R2 = CO2Me | 4p | 63 |
| 4 | R1 = Cl, R2 = Cl | 4q | 77 |
| 5 | R1 = Cl, R2 = H | 4r | 84 |
| 6 | R1 = F, R2 = H | 4s | 72 |
| 7 |  | 4t | 49 |
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Preparation of Isothiocyanates 4a–m
3.3. General Procedure for the Preparation of Isothiocyanates 4n–t
3.4. Characterization Data
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pedras, M.S.C.; Zheng, Q.-A.; Gadagi, R.S. The first naturally occurring aromatic isothiocyanates, rapalexins a and b, are cruciferous phytoalexins. Chem. Commun. 2007, 368–370. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Q.-H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef]
- Adsule, S.; Banerjee, S.; Ahmed, F.; Padhye, S.; Sarkar, F.H. Hybrid anticancer agents: Isothiocyanate-progesterone conjugates as chemotherapeutic agents and insights into their cytotoxicities. Bioorg. Med. Chem. Lett. 2010, 20, 1247–1251. [Google Scholar] [CrossRef]
- Galuppo, M.; Nicola, G.; Iori, R.; Dell'Utri, P.; Bramanti, P.; Mazzon, E. Antibacterial activity of glucomoringin bioactivated with myrosinase against two important pathogens affecting the health of long-term patients in hospitals. Molecules 2013, 18, 14340–14348. [Google Scholar] [CrossRef]
- Wu, H.; Feng, J.-T.; Lin, K.-C.; Zhang, X. Synthesis and herbicidal activity of substituted pyrazole isothiocyanates. Molecules 2012, 17, 12187–12196. [Google Scholar] [CrossRef]
- Azaiez, I.; Meca, G.; Manyes, L.; Fernández-Franzón, M. Antifungal activity of gaseous allyl, benzyl and phenyl isothiocyanate in vitro and their use for fumonisins reduction in bread. Food Control 2013, 32, 428–434. [Google Scholar] [CrossRef]
- Santos, J.C.; Faroni, L.R.A.; Sousa, A.H.; Guedes, R.N.C. Fumigant toxicity of allyl isothiocyanate to populations of the red flour beetle tribolium castaneum. J. Stored Prod. Res. 2011, 47, 238–243. [Google Scholar] [CrossRef]
- Sun, Z.-H.; Huang, W.; Gong, Y.-Y.; Lan, J.; Liu, X.-H.; Weng, J.-Q.; Li, Y.-S.; Tan, C.-X. Synthesis and herbicidal activity of new 1,3,4-thiadizols sulfourea derivative. Chin. J. Org. Chem. 2013, 33, 2612–2617. [Google Scholar] [CrossRef]
- Mukerjee, A.K.; Ashare, R. Isothiocyanates in the chemistry of heterocycles. Chem. Rev. 1991, 91, 1–24. [Google Scholar] [CrossRef]
- Hemdan, M.M.; Fahmy, A.F.; Ali, N.F.; Hegazi, E.; Abd-Elhaleem, A. Synthesis of some new heterocycles derived from phenylacetyl isothiocyanate. Chin. J. Chem. 2008, 26, 388–391. [Google Scholar] [CrossRef]
- Wu, X.-L.; Zhu, C.-F.; Lv, Z.-D.; Wei, C.-S.; Liao, X.-C. Synthesis of sulfur ethers containing 1,3,4-oxadiazole and 1,3,4-thiadiazole. Chin. J. Org. Chem. 2011, 31, 824–831. [Google Scholar]
- Karpyak, V.; Obushak, M.; Ganushchak, M. Synthesis of 2-(2-R1-hydrazino)-5-(R2-benzyl)-2-thiazolines on the basis of meerweins arylation products of allyl isothiocyanate. Molecules 2003, 8, 263–268. [Google Scholar] [CrossRef]
- Nath, J.; Ghosh, H.; Yella, R.; Patel, B.K. Molecular iodine mediated preparation of isothiocyanates from dithiocarbamic acid salts. Eur. J. Org. Chem. 2009, 2009, 1849–1851. [Google Scholar]
- Wong, R.; Dolman, S.J. Isothiocyanates from tosyl chloride mediated decomposition of in situ generated dithiocarbamic acid salts. J. Org. Chem. 2007, 72, 3969–3971. [Google Scholar] [CrossRef]
- Munch, H.; Hansen, J.S.; Pittelkow, M.; Christensen, J.B.; Boas, U. A new efficient synthesis of isothiocyanates from amines using di-tert-butyl dicarbonate. Tetrahedron Lett. 2008, 49, 3117–3119. [Google Scholar] [CrossRef]
- Sun, N.; Li, B.; Shao, J.-P.; Mo, W.-M.; Hu, B.-X.; Shen, Z.-L.; Hu, X.-Q. A general and facile one-pot process of isothiocyanates from amines under aqueous conditions. Beilstein J. Org. Chem. 2012, 8, 61–70. [Google Scholar] [CrossRef]
- Liu, P.-F.; Li, C.-Y.; Zhang, J.-W.; Xu, X.-Y. Facile and versatile synthesis of alkyl and aryl isothiocyanates by using triphosgene and cosolvent. Synth. Commun. 2013, 43, 3342–3351. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Ma, H.-Z.; Han, C.; Xi, H.-T.; Meng, Q.; Chen, X.; Sun, X.-Q. Synthesis of isothiocyanates by reaction of amines with phenyl chlorothionoformate via one-pot or two-step process. Synthesis 2013, 45, 1667–1674. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.-H.; Tan, Y.-J. A simple and straightforward synthesis of phenyl isothiocyanates, symmetrical and unsymmetrical thioureas under ball milling. RSC Adv. 2013, 3, 16940–16944. [Google Scholar] [CrossRef]
- Pronin, S.V.; Reiher, C.A.; Shenvi, R.A. Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles and amines. Nature 2013, 501, 195–199. [Google Scholar] [CrossRef]
- Zhong, B.; Al-Awar, R.S.; Shih, C.; Grimes, J.H.; Vieth, M.; Hamdouchi, C. Novel route to the synthesis of 4-quinolyl isothiocyanates. Tetrahedron Lett. 2006, 47, 2161–2164. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Yusof, M.S.M.; Amin, N.M. Anti-amoebic properties of carbonyl thiourea derivatives. Molecules 2014, 19, 5191–5204. [Google Scholar] [CrossRef]
- Burkett, B.A.; Fu, P.; Hewitt, R.J.; Ng, S.L.; Toh, J.D.W. Purification-free, small-scale synthesis of isothiocyanates by reagentless fragmentation of polymer-supported 1,4,2-oxathiazoles. Eur. J. Org. Chem. 2014, 2014, 1053–1058. [Google Scholar]
- Kobayashi, K.; Kobayashi, A.; Ezaki, K. One-pot synthesis of 2-sulfanyl-3-sulfinyl(or sulfonyl)-1H-indoles via cyclization of 1-isothiocyanato-2-[sulfinyl(or sulfonyl)methyl]benzenes with sodium hydride. Tetrahedron 2013, 69, 7936–7942. [Google Scholar] [CrossRef]
- Fujiwara, S.; Shin-Ike, T.; Sonoda, N.; Aoki, M.; Okada, K.; Miyoshi, N.; Kambe, N. Novel selenium catalyzed synthesis of isothiocyanates from isocyanides and elemental sulfur. Tetrahedron Lett. 1991, 32, 3503–3506. [Google Scholar] [CrossRef]
- Larsen, C.; Steliou, K.; Harpp, D.N. Organic sulfur chemistry. 25. Thiocarbonyl transfer reagents. J. Org. Chem. 1978, 43, 337–339. [Google Scholar] [CrossRef]
- Kim, S.; Yi, K.Y. Di-2-pyridyl thionocarbonate. A new reagent for the preparation of isothiocyanates and carbodiimides. Tetrahedron Lett. 1985, 26, 1661–1664. [Google Scholar] [CrossRef]
- Le Count, D.J.; Dewsbury, D.J.; Grundy, W. An improved synthesis of pyridyl isothiocyanates and thioureas. Synthesis 1977, 582–583. [Google Scholar] [CrossRef]
- Ding, F.-Z.; Smith, J.M.; Wang, H.-B. First-principles calculation of pKa values for organic acids in nonaqueous solution. J. Org. Chem. 2009, 74, 2679–2691. [Google Scholar] [CrossRef]
- Streitwieser, A.; Kim, Y.-J. Ion pair basicity of some amines in THF: Implications for ion pair acidity scales. J. Am. Chem. Soc. 2000, 122, 11783–11786. [Google Scholar] [CrossRef]
- Cecchi, L.; de Sarlo, F.; Machetti, F. 1,4-diazabicyclo [2.2.2]octane (DABCO) as an efficient reagent for the synthesis of isoxazole derivatives from primary nitro compounds and dipolarophiles: The role of the base. Eur. J. Org. Chem. 2006, 2006, 4852–4860. [Google Scholar] [CrossRef]
- Perrin, D.D. Dissociation Constants of Organic Bases in Aqueous Solution; Butterworths: London, UK, 1965. [Google Scholar]
- Haynes, W.M. Crc Handbook of Chemistry and Physics, 95th ed; Lide, D.R., Ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Nair, V.; Kim, K.H. The reaction of 1-azirines with 2-pyridyl isothiocyanate: Possible approaches to benzodiazepine and benzodiazepine derivatives. J. Heterocycl. Chem. 1976, 13, 873–876. [Google Scholar] [CrossRef]
- L'abbé, G.; Allewaert, K.; Toppet, S. A rare case of three rearrangements during the cycloaddition-elimination reaction of 4-methyl-5-phenylimino-Δ2-1,2,3,4-thiatriazoline with 2-pyridyl isothiocyanate. J. Heterocycl. Chem. 1988, 25, 1459–1462. [Google Scholar] [CrossRef]
- Ramachandran, S.; Shahul, H.P.; Srivastava, A.; Shanbhag, G.; Morayya, S.; Rautela, N.; Awasthy, D.; Kavanagh, S.; Bharath, S.; Reddy, J.; et al. N-Aryl-2-aminobenzimidazoles: Novel, efficacious, antimalarial lead compounds. J. Med. Chem. 2014, 57, 6642–6652. [Google Scholar] [CrossRef]
- Altenbach, R.J.; Bai, H.; Brioni, J.D.; Carroll, W.A.; Gopalakrishnan, M.; Gregg, R.J.; Holladay, M.W.; Huang, P.P.; Kincaid, J.F.; Kort, M.E.; et al. Potassium Channel Openers. U.S. Patent 2002/0028836A1; filed 05 February 2001, and issued 07 March 2002,
- Sample Availability: Samples of the compounds 4a–t are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.; Liu, R.-Q.; Liu, K.-C.; Li, Q.-B.; Li, Q.-Y.; Liu, S.-Z. A One-Pot Approach to Pyridyl Isothiocyanates from Amines. Molecules 2014, 19, 13631-13642. https://doi.org/10.3390/molecules190913631
Zhang H, Liu R-Q, Liu K-C, Li Q-B, Li Q-Y, Liu S-Z. A One-Pot Approach to Pyridyl Isothiocyanates from Amines. Molecules. 2014; 19(9):13631-13642. https://doi.org/10.3390/molecules190913631
Chicago/Turabian StyleZhang, Hao, Rui-Quan Liu, Ke-Chang Liu, Qi-Bo Li, Qing-Yang Li, and Shang-Zhong Liu. 2014. "A One-Pot Approach to Pyridyl Isothiocyanates from Amines" Molecules 19, no. 9: 13631-13642. https://doi.org/10.3390/molecules190913631
APA StyleZhang, H., Liu, R. -Q., Liu, K. -C., Li, Q. -B., Li, Q. -Y., & Liu, S. -Z. (2014). A One-Pot Approach to Pyridyl Isothiocyanates from Amines. Molecules, 19(9), 13631-13642. https://doi.org/10.3390/molecules190913631